Cordycepin Modulates Microglial M2 Polarization Coupled with Mitochondrial Metabolic Reprogramming by Targeting HKII and PDK2
- PMID: 38889331
- PMCID: PMC11336950
- DOI: 10.1002/advs.202304687
Cordycepin Modulates Microglial M2 Polarization Coupled with Mitochondrial Metabolic Reprogramming by Targeting HKII and PDK2
Abstract
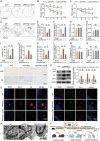
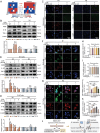
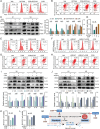
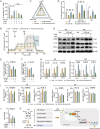
The microenvironment mediated by the microglia (MG) M1/M2 phenotypic switch plays a decisive role in the neuronal fate and cognitive function of Alzheimer's disease (AD). However, the impact of metabolic reprogramming on microglial polarization and its underlying mechanism remains elusive. This study reveals that cordycepin improved cognitive function and memory in APP/PS1 mice, as well as attenuated neuronal damage by triggering MG-M2 polarization and metabolic reprogramming characterized by increased OXPHOS and glycolysis, rather than directly protecting neurons. Simultaneously, cordycepin partially alleviates mitochondrial damage in microglia induced by inhibitors of OXPHOS and glycolysis, further promoting MG-M2 transformation and increasing neuronal survival. Through confirmation of cordycepin distribution in the microglial mitochondria via mitochondrial isolation followed by HPLC-MS/MS techniques, HKII and PDK2 are further identified as potential targets of cordycepin. By investigating the effects of HKII and PDK2 inhibitors, the mechanism through which cordycepin targeted HKII to elevate ECAR levels in the glycolysis pathway while targeting PDK2 to enhance OCR levels in PDH-mediated OXPHOS pathway, thereby inducing MG-M2 polarization, promoting neuronal survival and exerting an anti-AD role is elucidated.
Keywords: HKII; PDK2; cordycepin; metabolic reprogramming; microglial polarization.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- a) Battaglini M., Marino A., Montorsi M., Carmignani A., Ceccarelli M. C., Ciofani G., Adv. Healthcare Mater. 2024, 13, e2304180; - PubMed
- b) Lan X., Han X., Li Q., Yang Q. W., Wang J., Nat. Rev. Neurol. 2017, 13, 420; - PMC - PubMed
- c) Plastira I., Bernhart E., Goeritzer M., Reicher H., Kumble V. B., Kogelnik N., Wintersperger A., Hammer A., Schlager S., Jandl K., Heinemann A., Kratky D., Malle E., Sattler W., J. Neuroinflamm. 2016, 13, 205; - PMC - PubMed
- d) Latta C. H., Sudduth T. L., Weekman E. M., Brothers H. M., Abner E. L., Popa G. J., Mendenhall M. D., Gonzalez‐Oregon F., Braun K., Wilcock D. M., J. Neuroinflamm. 2015, 12, 41. - PMC - PubMed
-
- a) Benzinger T. L., Blazey T., Jack C. R. Jr., Koeppe R. A., Su Y., Xiong C., Raichle M. E., Snyder A. Z., Ances B. M., Bateman R. J., Cairns N. J., Fagan A. M., Goate A., Marcus D. S., Aisen P. S., Christensen J. J., Ercole L., Hornbeck R. C., Farrar A. M., Aldea P., Jasielec M. S., Owen C. J., Xie X., Mayeux R., Brickman A., McDade E., Klunk W., Mathis C. A., Ringman J., Thompson P. M., et al., Proc. Natl. Acad. Sci. USA 2013, 110, E4502; - PMC - PubMed
- b) Mosconi L., Eur. J. Nucl. Med. Mol. Imag. 2005, 32, 486. - PubMed
-
- a) Chen Z., Liu M., Li L., Chen L., J. Cell. Physiol. 2018, 233, 2839; - PubMed
- b) Szelechowski M., Amoedo N., Obre E., Léger C., Allard L., Bonneu M., Claverol S., Lacombe D., Oliet S., Chevallier S., Masson G. L., Rossignol R., Sci. Rep. 2018, 8, 3953; - PMC - PubMed
- c) Vallée A., Lecarpentier Y., Guillevin R., Vallée J. N., Rev. Neurosci. 2018, 29, 547. - PubMed
MeSH terms
Substances
Grants and funding
- LJKZ0775/Major Basic Research Project of the Natural Science Foundation of the Jiangsu Higher Education Institutions
- 2018225089/Key Research and Development Program of Liaoning Province
- 2019-BS-289/Doctoral Start-up Foundation of Liaoning Province
- 81901309/National Natural Science Foundation of China
- 123-3110210138/The Science and Technology Funding Project to support the high-quality development of China Medical University
LinkOut - more resources
Full Text Sources
