Interleukin-6 Signaling Blockade Induces Regulatory Plasmablasts in Neuromyelitis Optica Spectrum Disorder
- PMID: 38889374
- PMCID: PMC11188987
- DOI: 10.1212/NXI.0000000000200266
Interleukin-6 Signaling Blockade Induces Regulatory Plasmablasts in Neuromyelitis Optica Spectrum Disorder
Abstract
Background and objectives: Interleukin-6 receptor antibodies (IL-6R Abs), including satralizumab, are increasingly used to prevent relapse for neuromyelitis optica spectrum disorder (NMOSD). However, the detailed mechanism of action of this treatment on the lymphocyte phenotype remains unclear. This study focused on B cells in patients with NMOSD, hypothesizing that IL-6R Ab enables B cells to acquire regulatory functions by producing the anti-inflammatory cytokine IL-10.
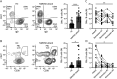
Methods: Peripheral blood mononuclear cells were stimulated in vitro to induce the expansion of B-cell subsets, double-negative B cells (DNs; CD19+ IgD-, CD27-) and plasmablasts (PBs; CD19+, CD27hi, CD38hi). Whole B cells, DNs, or PBs were isolated after culture with IL-6R Ab, and IL-10 expression was quantified using quantitative PCR and a cytometric bead array. RNA sequencing was performed to identify the marker of regulatory PBs induced by IL-6R Ab.
Results: DNs and PBs were observed to expand in patients with NMSOD during the acute attacks. In the in vitro model, IL-6R Ab increased IL-10 expression in B cells. Notably, IL-10 expression increased in PBs but not in DNs. Using RNA sequencing, CD200 was identified as a marker of regulatory PBs among the differentially expressed upregulated genes. CD200+ PBs produced more IL-10 than CD200- PBs. Furthermore, patients with NMOSD who received satralizumab had a higher proportion of CD200+ PBs than patients during the acute attacks.
Discussion: Treatment with IL-6 signaling blockade elicited a regulatory phenotype in B cells and PBs. CD200+ PBs may be a marker of treatment responsiveness in the context of NMOSD pathophysiology.
Conflict of interest statement
The authors report no relevant disclosures. Go to
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
