Using the Progression Independent of Relapse Activity Framework to Unveil the Pathobiological Foundations of Multiple Sclerosis
- PMID: 38889384
- PMCID: PMC11226318
- DOI: 10.1212/WNL.0000000000209444
Using the Progression Independent of Relapse Activity Framework to Unveil the Pathobiological Foundations of Multiple Sclerosis
Abstract
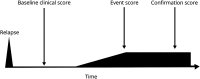
Progression independent of relapse activity (PIRA), a recent concept to formalize disability accrual in multiple sclerosis (MS) independent of relapses, has gained popularity as a potential clinical trial outcome. We discuss its shortcomings and appraise the challenges of implementing it in clinical settings, experimental trials, and research. The current definition of PIRA assumes that acute inflammation, which can manifest as a relapse, and neurodegeneration, manifesting as progressive disability accrual, can be disentangled by introducing specific time windows between the onset of relapses and the observed increase in disability. The term PIRMA (progression independent of relapse and MRI activity) was recently introduced to indicate disability accrual in the absence of both clinical relapses and new brain and spinal cord MRI lesions. Assessing PIRMA in clinical practice is highly challenging because it necessitates frequent clinical assessments and brain and spinal cord MRI scans. PIRA is commonly assessed using Expanded Disability Status Scale, a scale heavily weighted toward motor disability, whereas a more granular assessment of disability deterioration, including cognitive decline, using composite measures or other tools, such as digital tools, would possess greater utility. Similarly, using PIRA as an outcome measure in randomized clinical trials is also challenging and requires methodological considerations. The underpinning pathobiology of disability accumulation, that is not associated with relapses, may encompass chronic active lesions (slowly expanding lesions and paramagnetic rim lesions), cortical lesions, brain and spinal cord atrophy, particularly in the gray matter, diffuse and focal microglial activation, persistent leptomeningeal enhancement, and white matter tract damage. We propose to use PIRA to understand the main determinant of disability accrual in observational, cohort studies, where regular MRI scans are not included, and introduce the term of "advanced-PIRMA" to investigate the contributions to disability accrual of the abovementioned processes, using conventional and advanced imaging. This is supported by the knowledge that MRI reflects the MS pathogenic mechanisms better than purely clinical descriptors. Any residual disability accrual, which remains unexplained after considering all these mechanisms with imaging, will highlight future research priorities to help complete our understanding of MS pathogenesis.
Conflict of interest statement
O. Ciccarelli is a NIHR Research Professor (RP-2017-08-ST2-004), over the last 2 years she has been a member of independent DSMB for Novartis, gave a teaching talk in a Merck local symposium, and contributed to an Advisory Board for Biogen, she is Deputy Editor of
Figures

References
-
- Kappos L, Butzkueven H, Wiendl H, et al. ; Tysabri Observational Program (TOP) Investigators. Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study. Mult Scler. 2018;24(7):963-973. doi:10.1177/1352458517709619 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
