Macrophage IL-1β mediates atrial fibrillation risk in diabetic mice
- PMID: 38889387
- PMCID: PMC11383594
- DOI: 10.1172/jci.insight.171102
Macrophage IL-1β mediates atrial fibrillation risk in diabetic mice
Abstract
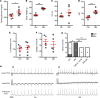
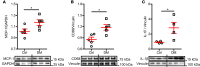
Diabetes mellitus (DM) is an independent risk factor for atrial fibrillation (AF). The mechanisms underlying DM-associated AF are unclear. AF and DM are both related to inflammation. We investigated whether DM-associated inflammation contributed to AF risk. Mice were fed with high-fat diet to induce type II DM and were subjected to IL-1β antibodies, macrophage depletion by clodronate liposomes, a mitochondrial antioxidant (mitoTEMPO), or a cardiac ryanodine receptor 2 (RyR2) stabilizer (S107). All tests were performed at 36-38 weeks of age. DM mice presented with increased AF inducibility, enhanced mitochondrial reactive oxygen species (mitoROS) generation, and activated innate immunity in the atria, as evidenced by enhanced monocyte chemoattractant protein-1 (MCP-1) expression, macrophage infiltration, and IL-1β levels. Signs of aberrant RyR2 Ca2+ leak were observed in the atria of DM mice. IL-1β neutralization, macrophage depletion, and exposure to mitoTEMPO and S107 significantly ameliorated the AF vulnerability in DM mice. Atrial overexpression of MCP-1 increased AF occurrence in normal mice through the same mechanistic signaling cascade as observed in DM mice. In conclusion, macrophage-mediated IL-1β contributed to DM-associated AF risk through mitoROS modulation of RyR2 Ca2+ leak.
Keywords: Arrhythmias; Cardiology; Diabetes; Inflammation; Macrophages.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

