Tumour-specific activation of a tumour-blood transport improves the diagnostic accuracy of blood tumour markers in mice
- PMID: 38889481
- PMCID: PMC11237870
- DOI: 10.1016/j.ebiom.2024.105178
Tumour-specific activation of a tumour-blood transport improves the diagnostic accuracy of blood tumour markers in mice
Abstract
Background: The accuracy of blood-based early tumour recognition is compromised by signal production at non-tumoral sites, low amount of signal produced by small tumours, and variable tumour production. Here we examined whether tumour-specific enhancement of vascular permeability by the particular tumour homing peptide, iRGD, which carries dual function of binding to integrin receptors overexpressed in the tumour vasculature and is known to promote extravasation via neuropilin-1 receptor upon site-specific cleavage, might be useful to improve blood-based tumour detection by inducing a yet unrecognised vice versa tumour-to-blood transport.
Methods: To detect an iRGD-induced tumour-to-blood transport, we examined the effect of intravenously injected iRGD on blood levels of α-fetoprotein (AFP) and autotaxin in several mouse models of hepatocellular carcinoma (HCC) or in mice with chronic liver injury without HCC, and on prostate-specific antigen (PSA) levels in mice with prostate cancer.
Findings: Intravenously injected iRGD rapidly and robustly elevated the blood levels of AFP in several mouse models of HCC, but not in mice with chronic liver injury. The effect was primarily seen in mice with small tumours and normal basal blood AFP levels, was attenuated by an anti-neuropilin-1 antibody, and depended on the concentration gradient between tumour and blood. iRGD treatment was also able to increase blood levels of autotaxin in HCC mice, and of PSA in mice with prostate cancer.
Interpretation: We conclude that iRGD induces a tumour-to-blood transport in a tumour-specific fashion that has potential of improving diagnosis of early stage cancer.
Funding: Deutsche Krebshilfe, DKTK, LOEWE-Frankfurt Cancer Institute.
Keywords: CEND-1; Early cancer detection; HCC; Tumour marker; iRGD; α fetoprotein.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests OW: Personal fees from Amgen, Bayer, BMS, Celgene, Daiicgi Sankyo, Eisai, Incyte, Ipsen, Merck, MSD, Novartis, Pierre Fabre, Roche, Servier; honoraria for lectures and/or presentations from Amgen, AstraZeneca, Bayer, BMS, Eisai, Ipsen, MSD, Novartis, Roche, Zentiva; support for attending meetings and/or travel: Abbvie, AstraZeneca, Bayer, BMS, Gilead, Ipsen, Medac, Merck, Pierre Fabre, Roche. SZ: Consultancy and/or speaker’s bureau: Abbvie, BioMarin, Boehringer Ingelheim, Gilead, GSK, Ipsen, Madrigal, Merck/MSD, NovoNordisk, SoBi. JUM: Grants or contracts from any entity, AstraZeneca, consulting fees: AstraZeneca, Roche, Ipsen, Eisai; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: AstraZeneca, Roche, Ipsen, Eisai. The other authors declare no conflicts of interest.
Figures

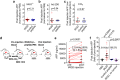
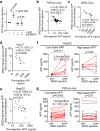

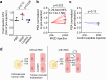
References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. - PubMed
-
- Llovet J.M., Kelley R.K., Villanueva A., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. - PubMed
-
- Debruyne E.N., Delanghe J.R. Diagnosing and monitoring hepatocellular carcinoma with alpha-fetoprotein: new aspects and applications. Clin Chim Acta. 2008;395:19–26. - PubMed
-
- Catalona W.J., Smith D.S., Ratliff T.L., et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Eng J Med. 1991;324:1156–1161. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

