Temporal and spatial progression of microstructural cerebral degeneration in ALS: A multicentre longitudinal diffusion tensor imaging study
- PMID: 38889523
- PMCID: PMC11231599
- DOI: 10.1016/j.nicl.2024.103633
Temporal and spatial progression of microstructural cerebral degeneration in ALS: A multicentre longitudinal diffusion tensor imaging study
Abstract
Objective: The corticospinal tract (CST) reveals progressive microstructural alterations in ALS measurable by DTI. The aim of this study was to evaluate fractional anisotropy (FA) along the CST as a longitudinal marker of disease progression in ALS.
Methods: The study cohort consisted of 114 patients with ALS and 110 healthy controls from the second prospective, longitudinal, multicentre study of the Canadian ALS Neuroimaging Consortium (CALSNIC-2). DTI and clinical data from a harmonized protocol across 7 centres were collected. Thirty-nine ALS patients and 61 controls completed baseline and two follow-up visits and were included for longitudinal analyses. Whole brain-based spatial statistics and hypothesis-guided tract-of-interest analyses were performed for cross-sectional and longitudinal analyses.
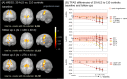
Results: FA was reduced at baseline and longitudinally in the CST, mid-corpus callosum (CC), frontal lobe, and other ALS-related tracts, with alterations most evident in the CST and mid-CC. CST and pontine FA correlated with functional impairment (ALSFRS-R), upper motor neuron function, and clinical disease progression rate. Reduction in FA was largely located in the upper CST; however, the longitudinal decline was greatest in the lower CST. Effect sizes were dependent on region, resulting in study group sizes between 17 and 31 per group over a 9-month interval. Cross-sectional effect sizes were maximal in the upper CST; whereas, longitudinal effect sizes were maximal in mid-callosal tracts.
Conclusions: Progressive microstructural alterations in ALS are most prominent in the CST and CC. DTI can provide a biomarker of cerebral degeneration in ALS, with longitudinal changes in white matter demonstrable over a reasonable observation period, with a feasible number of participants, and within a multicentre framework.
Keywords: Amyotrophic Lateral Sclerosis; DTI; Diffusion tensor imaging; MRI; Magnetic resonance imaging; Motor cortex; Multicentre study.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Agosta F., Rocca M.A., Valsasina P., et al. A longitudinal diffusion tensor MRI study of the cervical cord and brain in amyotrophic lateral sclerosis patients. J. Neurol. Neurosurg. Psychiatry. 2009;80:53–55. - PubMed
-
- Arnold G., Boone K.B., Lu P., et al. Sensitivity and specificity of finger tapping test scores for the detection of suspect effort. Clin. Neuropsychol. 2005;19:105–120. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

