Bortezomib, thalidomide, and dexamethasone with or without daratumumab and followed by daratumumab maintenance or observation in transplant-eligible newly diagnosed multiple myeloma: long-term follow-up of the CASSIOPEIA randomised controlled phase 3 trial
- PMID: 38889735
- PMCID: PMC12375812
- DOI: 10.1016/S1470-2045(24)00282-1
Bortezomib, thalidomide, and dexamethasone with or without daratumumab and followed by daratumumab maintenance or observation in transplant-eligible newly diagnosed multiple myeloma: long-term follow-up of the CASSIOPEIA randomised controlled phase 3 trial
Abstract
Background: CASSIOPEIA part 1 demonstrated superior depth of response and prolonged progression-free survival with daratumumab in combination with bortezomib, thalidomide, and dexamethasone (D-VTd) versus bortezomib, thalidomide, and dexamethasone (VTd) alone as an induction and consolidation regimen in transplant-eligible patients newly diagnosed with myeloma. In CASSIOPEIA part 2, daratumumab maintenance significantly improved progression-free survival and increased minimal residual disease (MRD)-negativity rates versus observation. Here, we report long-term study outcomes of CASSIOPEIA.
Methods: CASSIOPEIA was a two-part, open-label, phase 3 trial of patients done at 111 European academic and community-based centres. Eligible patients were aged 18-65 years with transplant-eligible newly diagnosed myeloma and an Eastern Cooperative Oncology Group performance status of 0-2. In part 1, patients were randomly assigned (1:1) to pre-transplant induction and post-transplant consolidation with D-VTd or VTd. Patients who completed consolidation and had a partial response or better were re-randomised (1:1) to intravenous daratumumab maintenance (16 mg/kg every 8 weeks) or observation for 2 years or less. An interactive web-based system was used for both randomisations, and randomisation was balanced using permuted blocks of four. Stratification factors for the first randomisation (induction and consolidation phase) were site affiliation, International Staging System disease stage, and cytogenetic risk status. Stratification factors for the second randomisation (maintenance phase) were induction treatment and depth of response in the induction and consolidation phase. The primary endpoint for the induction and consolidation phase was the proportion of patients who achieved a stringent complete response after consolidation; results for this endpoint remain unchanged from those reported previously. The primary endpoint for the maintenance phase was progression-free survival from second randomisation. Efficacy evaluations in the induction and consolidation phase were done on the intention-to-treat population, which included all patients who underwent first randomisation, and efficacy analyses in the maintenance phase were done in the maintenance-specific intention-to-treat population, which included all patients who were randomly assigned at the second randomisation. This analysis represents the final data cutoff at the end of the study. The trial is registered with ClinicalTrials.gov, NCT02541383.
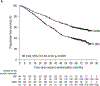
Findings: Between Sept 22, 2015 and Aug 1, 2017, 1085 patients were randomly assigned to D-VTd (n=543) or VTd (n=542); between May 30, 2016 and June 18, 2018, 886 were re-randomised to daratumumab maintenance (n=442) or observation (n=444). At the clinical cutoff date, Sept 1, 2023, median follow-up was 80·1 months (IQR 75·7-85·6) from first randomisation and 70·6 months (66·4-76·1) from second randomisation. Progression-free survival from second randomisation was significantly longer in the daratumumab maintenance group than the observation-alone group (median not reached [95% CI 79·9-not estimable (NE)] vs 45·8 months [41·8-49·6]; HR 0·49 [95% CI 0·40-0·59]; p<0·0001); benefit was observed with D-VTd with daratumumab maintenance versus D-VTd with observation (median not reached [74·6-NE] vs 72·1 months [52·8-NE]; 0·76 [0·58-1·00]; p=0·048) and VTd with daratumumab maintenance versus VTd with observation (median not reached [66·9-NE] vs 32·7 months [27·2-38·7]; 0·34 [0·26-0·44]; p<0·0001).
Interpretation: The long-term follow-up results of CASSIOPEIA show that including daratumumab in both the induction and consolidation phase and the maintenance phase led to superior progression-free survival outcomes. Our results confirm D-VTd induction and consolidation as a standard of care, and support the option of subsequent daratumumab monotherapy maintenance, for transplant-eligible patients with newly diagnosed multiple myeloma.
Funding: Intergroupe Francophone du Myélome, Dutch-Belgian Cooperative Trial Group for Hematology Oncology, and Janssen Research & Development.
Copyright © 2024 Elsevier Ltd. All rights reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Declaration of interests PM served on advisory boards for and received honoraria from Janssen, Bristol Myers Squibb, Amgen, Takeda, AbbVie, Sanofi, and Pfizer. CH received honoraria from Janssen, Bristol Myers Squibb, Amgen, AbbVie, and Pfizer. AP served in a consulting or advisory role for Bristol Myers Squibb, Janssen, and Pfizer; and received research funding from Bristol Myers Squibb, Sanofi, and Takeda. BA received research funding (paid to institution) from Bristol Myers Squibb; received honoraria from Janssen, Bristol Myers Squibb, Sanofi, and GSK; received travel funding from Janssen, Bristol Myers Squibb, and Sanofi; and served on an advisory board for Janssen, Bristol Myers Squibb, and Takeda. KB has a direct financial relationship with Janssen, Bristol Myers Squibb, Amgen, Sanofi, and Pfizer. SZ received research support from Janssen and Takeda; and served on advisory boards for Janssen, Bristol Myers Squibb, Sanofi, Oncopeptides, Amgen, and Takeda. MD received honoraria from and served in a consulting or advisory role for Amgen, Bristol Myers Squibb, Janssen, Sanofi, and Stemline; and received research funding from Janssen. CS received consulting fees or honoraria from Takeda, Bristol Myers Squibb, Janssen, Amgen, Sanofi, and Pfizer. MMo received honoraria from Adaptive Biotechnologies, Amgen, Bristol Myers Squibb, Celgene, Janssen, Takeda, Novartis, and Sanofi; and received research funding from Celgene, Janssen, and Sanofi. LK received honoraria from, served on advisory boards for, or received travel funding from AbbVie, Amgen, Janssen, Celgene/Bristol Myers Squibb, Pfizer, Sanofi, and Takeda. MMa received honoraria from and served in a consulting or advisory role for Janssen, Bristol Myers Squibb/Celgene, Sanofi, and Takeda; received research funding from Janssen and Takeda; and received travel, accommodations, or expenses from Janssen and Sanofi. FO-P served on boards for Janssen, Sanofi, and Pfizer. MR served on an advisory board for Janssen and Pfizer; received research funding from Bristol Myers Squibb, Janssen, and Sanofi; gave lectures for Amgen, Bristol Myers Squibb, Janssen, and Takeda; and had travel, accommodations, or other expenses paid or reimbursed by Amgen, Bristol Myers Squibb, GSK, Janssen, Pfizer, Sanofi, and Takeda. JMSdC served on an advisory board and received honoraria from Janssen, Sanofi, GSK, and AbbVie; and received accommodations from Pfizer and Amgen. NWCJvdD received research support from Janssen, Amgen, Celgene, Novartis, Cellectis, and Bristol Myers Squibb; and serves on advisory boards for Janssen, Amgen, Celgene, Bristol Myers Squibb, Sanofi, Takeda, Roche, Novartis, Bayer, Adaptive, Merck, Pfizer, AbbVie, and Servier (all paid to institution). AB served on advisory boards for Janssen, Sanofi, Amgen, and Bristol Myers Squibb. CT served on an advisory board for and received honoraria from Janssen. NM served on an advisory board for Janssen. M-CV received travel support from Janssen and Sanofi. M-DL received travel expenses from Janssen. WH is an employee of Cytel and is a contractor for Johnson & Johnson. JW, CdB, MR, VV, RC, and JV are employees of Janssen and hold stock in Johnson & Johnson. AT is an employee of Janssen. JC served on advisory boards for Sanofi and Bristol Myers Squibb; served as a consultant for Janssen, Sanofi, Bristol Myers Squibb, Pfizer, and Adaptive; received research support from Sanofi and Bristol Myers Squibb; and received travel support from Janssen, Sanofi, Bristol Myers Squibb, and Pfizer. PS served on an advisory board for Amgen, Bristol Myers Squibb, Celgene, Janssen, Karyopharm, and Pfizer; and received research funding from Amgen, Bristol Myers Squibb, Celgene, Janssen, and Karyopharm. All other authors declare no competing interests.
Figures




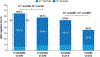

References
-
- de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 2011; 186(3): 1840–8. - PubMed
-
- Lammerts van Bueren J, Jakobs D, Kaldenhoven N, et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood 2014; 124(21): 3474.
-
- Overdijk MB, Jansen JH, Nederend M, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcgamma receptor-mediated cross-linking. J Immunol 2016; 197(3): 807–13. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

