Therapeutic options for neurocardiogenic syncope: a meta-analysis of randomised trials with and without blinding
- PMID: 38890128
- PMCID: PMC11191821
- DOI: 10.1136/openhrt-2024-002669
Therapeutic options for neurocardiogenic syncope: a meta-analysis of randomised trials with and without blinding
Abstract
Background: Neurocardiogenic syncope is a common condition with significant associated psychological and physical morbidity. The effectiveness of therapeutic options for neurocardiogenic syncope beyond placebo remains uncertain.
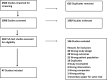
Methods: The primary endpoint was the risk ratio (RR) of spontaneously recurring syncope following any therapeutic intervention. We also examined the effect of blinding on treatment efficacy. We identified all randomised trials which evaluated the effect of any pharmacological, device-based or supportive intervention on patients with a history of syncope. A systematic search was conducted on Medline, Embase, PubMed databases and Cochrane Central Register for Controlled Trials from 1950 to 25 April 2023. Event rates, their RRs and 95% CIs were calculated, and a random-effects meta-analysis was conducted for each intervention. Data analysis was performed in R using RStudio.
Results: We identified 47 eligible trials randomising 3518 patients. Blinded trials assessing syncope recurrence were neutral for beta blockers, fludrocortisone and conventional dual-chamber pacing but were favourable for selective serotonin reuptake inhibitors (SSRIs) (RR 0.40, 95% CI 0.26 to 0.63, p<0.001), midodrine (RR 0.70, 95% CI 0.53 to 0.94, p=0.016) and closed-loop stimulation (CLS) pacing (RR 0.15, 95% CI 0.07 to 0.35, p<0.001). Unblinded trials reported significant benefits for all therapy categories other than beta blockers and consistently showed larger benefits than blinded trials.
Conclusions: Under blinded conditions, SSRIs, midodrine and CLS pacing significantly reduced syncope recurrence. Future trials for syncope should be blinded to avoid overestimating treatment effects.
Prospero registration number: CRD42022330148.
Keywords: Meta-Analysis; Pacemaker, Artificial; Quality of Health Care; SYNCOPE.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
