Expression of the tumor antigens NY-ESO-1, tyrosinase, MAGE-A3, and TPTE in pediatric and adult melanoma: a retrospective case control study
- PMID: 38890171
- PMCID: PMC11329550
- DOI: 10.1007/s00428-024-03846-0
Expression of the tumor antigens NY-ESO-1, tyrosinase, MAGE-A3, and TPTE in pediatric and adult melanoma: a retrospective case control study
Abstract
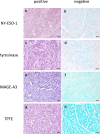
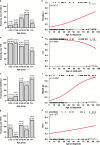
Tumor-associated antigens (TAAs) are potential targets for T cell-based immunotherapy approaches in cutaneous melanoma. BNT111, an investigational lipoplex-formulated mRNA-based therapeutic cancer vaccine encoding melanoma TAAs NY-ESO-1, tyrosinase, MAGE-A3, and TPTE, is undergoing clinical testing in adults. Expression of these TAAs in pediatric melanoma is unclear but is a prerequisite for feasibility of this treatment approach in children with melanoma. Our main objective was to characterize expression of those TAAs in pediatric melanomas compared to control cohorts. In this retrospective case control study, protein and transcript expression of NY-ESO-1, tyrosinase, MAGE-A3, and TPTE were analyzed in a cohort of 25 pediatric melanomas, 31 melanomas of young adults, 29 adult melanomas, and 30 benign melanocytic nevi in children using immunohistochemical staining and digital pathology (QuPath) and reverse transcription quantitative PCR. Based on IHC analysis, pediatric melanomas expressed tyrosinase (100.0%), TPTE (44.0%), MAGE-A3 (12.0%), and NY-ESO-1 (8.0%). Young adult melanomas expressed tyrosinase (96.8%), NY-ESO-1 (19.4%), MAGE-A3 (19.4%), and TPTE (3.2%). Adult melanomas expressed tyrosinase (86.2%), MAGE-A3 (75.9%), NY-ESO-1 (48.3%), and TPTE (48.3%). Childhood melanocytic nevi only expressed tyrosinase (93.3%). Expression prevalence of individual TAAs did not differ between subtypes of pediatric melanoma, and no association with prognosis was found. All four TAAs were expressed in pediatric melanoma, albeit NY-ESO-1 and MAGE-A3 to a lesser extent than in adult melanoma. These data support the possibility of investigating vaccines targeting these TAAs for the treatment of pediatric melanoma.
Keywords: Cancer vaccine; Pediatric melanoma; Prognostic factor; TAA.
© 2024. The Author(s).
Conflict of interest statement
SF: SF received institutional funding from BioNTech SE in relation to the submitted work, received personal honoraria from Kyowa Kirin and Recordati Rare Diseases (speaker’s honoraria), as well as institutional grants from Neracare and SkylineDX outside the submitted work.
OTP: OTP has no conflict of interest to declare.
MH: MH has no conflict of interest to declare.
VA: VA has no conflict of interest to declare.
CMS: CMS has no conflict of interest to declare.
CS: CS reports institutional grants from Illumina and research grants from BMS Stiftung Immunonkologie and Westdeutsche Studiengruppe GmbH outside the submitted work.
AL: AL has no conflict of interest to declare.
MA: MA has no conflict of interest to declare.
HW: HW has no conflict of interest to declare.
EB: EB has no conflict of interest to declare.
MK: MK has no conflict of interest to declare.
DS: DS has no conflict of interest to declare.
KC: KC is an employee at BioNTech and holds securities from BioNTech SE.
IB: IB is an employee at BioNTech and holds securities from BioNTech SE.
CF: CF is an employee at BioNTech and holds securities from BioNTech SE.
MP: MP is an employee at BioNTech and holds securities from BioNTech SE.
ML: ML is an employee at BioNTech and holds securities from BioNTech SE.
PB: PB is an employee at BioNTech and holds securities from BioNTech SE.
ÖT: ÖT is co-founder, management board member, and employee at BioNTech. ÖT has a leadership role at the Helmholtz Institute HI-TRON Mainz and is a founding member of TRON Translational Oncology Mainz. ÖT holds a professorship at the Johannes Gutenberg University. ÖT is an inventor on patents and patent applications related to RNA technology. ÖT holds securities from BioNTech SE.
US: US is co-founder, management board member, and employee at BioNTech. US has a leadership role at the Helmholtz Institute HI-TRON Mainz and is a founding member of TRON Translational Oncology Mainz. He holds a professorship at the Johannes Gutenberg University as professor in 2022 and was awarded with German Future Prize 2021. US is an inventor on patents and patent applications related to RNA technology. US holds securities from BioNTech SE.
LF: LF has/had advisory roles for Novartis, Sanofi, Philogen, and Bristol-Myers Squibb. LF received research support by Hookipa Pharma, Mundipharma and the Novartis Foundation. All outside the submitted work.
IBB: IBB received institutional funding from BioNTech SE in relation to the submitted work. IBB had advisory roles for Alexion outside the submitted work.
Figures


References
-
- Ferrari A, Brecht IB, Gatta G, Schneider DT, Orbach D, Cecchetto G et al (2019) Defining and listing very rare cancers of paediatric age: consensus of the Joint Action on Rare Cancers in cooperation with the European Cooperative Study Group for Pediatric Rare Tumors. Eur J Cancer 110:120–126. 10.1016/j.ejca.2018.12.031 10.1016/j.ejca.2018.12.031 - DOI - PubMed
-
- Achajew A, Brecht IB, Radespiel-Tröger M, Meyer M, Metzler M, Bremensdorfer C, Spix C, Erdmann F, Schneider DT, Abele M (2022) Rare pediatric tumors in Germany - not as rare as expected: a study based on data from the Bavarian Cancer Registry and the German Childhood Cancer Registry. Eur J Pediatr 181(7):2723–2730. 10.1007/s00431-022-04484-x 10.1007/s00431-022-04484-x - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

