Model-Informed Development of a Cost-Saving Dosing Regimen for Sacituzumab Govitecan
- PMID: 38890221
- PMCID: PMC11393053
- DOI: 10.1007/s11523-024-01075-8
Model-Informed Development of a Cost-Saving Dosing Regimen for Sacituzumab Govitecan
Abstract
Background: The antibody-drug conjugate sacituzumab govitecan is approved for metastatic triple-negative breast cancer and has shown promising results in various other types of cancer. Its costs may limit patient access to this novel effective treatment modality.
Objective: The purpose of this study was to develop an evidence-based rational dosing regimen that results in targeted drug exposure within the therapeutic range while minimizing financial toxicity, to improve treatment access.
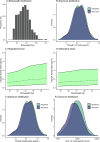
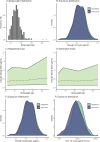
Patients and methods: Exposure equivalent dosing strategies were developed based on pharmacokinetic modeling and simulation by using the published pharmacokinetic model developed by the license holder. The alternative dose was based on the principle of using complete vials to prevent spillage and on the established non-linear relationship between body weight and systemic exposure. Equivalent exposure compared to the approved dosing regimen of 10 mg/kg was aimed for. Equivalent exposure was conservatively defined as calculated geometric mean ratios within the 0.9-1.11 boundaries for area under the concentration-time curve (AUC), trough concentration (Ctrough) and maximum concentration (Cmax) of the alternative dosing regimen compared to the approved dosing regimen. Since different vial sizes are available for the European Union (EU) and United States (US) market, because body weight distributions differ between these populations, we performed our analysis for both scenarios.
Results: Dosing regimens of sacituzumab govitecan for the EU (< 50 kg: 400 mg, 50-80 kg: 600 mg, and > 80 kg: 800 mg) and US population (< 40 kg: 360 mg, 40-65 kg: 540 mg, 65-90 kg: 720 mg, and > 90 kg: 900 mg) were developed, based on weight bands. The geometric mean ratios for all pharmacokinetic outcomes were within the predefined equivalence boundaries, while the quantity of drug used was 21.5% and 19.0% lower for the EU and US scenarios, respectively.
Conclusions: With the alternative dosing proposal, an approximately 20% reduction in drug expenses for sacituzumab govitecan can be realized while maintaining an equivalent and more evenly distributed exposure throughout the body weight range, without notable increases in pharmacokinetic variability.
© 2024. The Author(s).
Conflict of interest statement
D.J.A.R. Moes, J.J.M.A. Hendrikx, H.J. Guchelaar, R.H.J. Mathijssen, J.L. Bakker, V.O. Dezentjé, N. de Rouw, N.P. van Erp, E.F. Smit, M.M. van den Heuvel, T.H. Oude Munnink, M. van Kats, S. Croes, J. R. Kroep, J. Zwaveling, and R. ter Heine declare no competing interest related to this work.
Figures
References
-
- Early Activity With Frontline Sacituzumab Govitecan/Pembro in Metastatic NSCLC [press release]. 2023, September 10. Available from: https://www.gilead.com/news-and-press/press-room/press-releases/2023/9/g....
-
- NICE says new triple negative advanced breast cancer drug too expensive for NHS use. 2022. Available from: https://www.nice.org.uk/news/article/nice-says-new-triple-negative-advan....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

