Repeat controlled human Plasmodium falciparum infections delay bloodstream patency and reduce symptoms
- PMID: 38890271
- PMCID: PMC11189388
- DOI: 10.1038/s41467-024-49041-2
Repeat controlled human Plasmodium falciparum infections delay bloodstream patency and reduce symptoms
Abstract
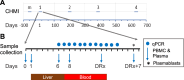
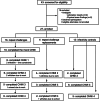
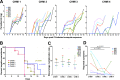
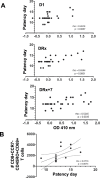
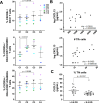
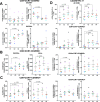
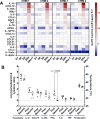
Resistance to clinical malaria takes years to develop even in hyperendemic regions and sterilizing immunity has rarely been observed. To evaluate the maturation of the host response against controlled repeat exposures to P. falciparum (Pf) NF54 strain-infected mosquitoes, we systematically monitored malaria-naïve participants through an initial exposure to uninfected mosquitoes and 4 subsequent homologous exposures to Pf-infected mosquitoes over 21 months (n = 8 males) (ClinicalTrials.gov# NCT03014258). The primary outcome was to determine whether protective immunity against parasite infection develops following repeat CHMI and the secondary outcomes were to track the clinical signs and symptoms of malaria and anti-Pf antibody development following repeat CHMI. After two exposures, time to blood stage patency increases significantly and the number of reported symptoms decreases indicating the development of clinical tolerance. The time to patency correlates positively with both anti-Pf circumsporozoite protein (CSP) IgG and CD8 + CD69+ effector memory T cell levels consistent with partial pre-erythrocytic immunity. IFNγ levels decrease significantly during the participants' second exposure to high blood stage parasitemia and could contribute to the decrease in symptoms. In contrast, CD4-CD8 + T cells expressing CXCR5 and the inhibitory receptor, PD-1, increase significantly after subsequent Pf exposures, possibly dampening the memory response and interfering with the generation of robust sterilizing immunity.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no completing interests. Material has been reviewed by the University of Maryland Institutional Review Board. There is no objection to its presentation and/or publication. The opinions or assertions expressed are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Uniformed Services University of the Health Sciences, Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25. This work was supported by NIAID of the National Institutes of Health (NIH) under award numbers R01AI110852 and U01AI110852; Clinical Trials.gov registration number, NCT03014258.
Figures

References
-
- World Health Organization, World Malaria Report 2022. (ed. World Health Organization) 1–293, https://www.who.int/publications/i/item/9789240064898 (2022).
-
- Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
Grants and funding
- U01AI110852/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI110852/AI/NIAID NIH HHS/United States
- R01AI110852/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI177698/AI/NIAID NIH HHS/United States
- U01 AI110852/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

