Penpulimab, an anti-PD-1 antibody, for heavily pretreated metastatic nasopharyngeal carcinoma: a single-arm phase II study
- PMID: 38890298
- PMCID: PMC11189389
- DOI: 10.1038/s41392-024-01865-6
Penpulimab, an anti-PD-1 antibody, for heavily pretreated metastatic nasopharyngeal carcinoma: a single-arm phase II study
Abstract
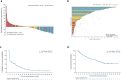
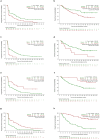
Penpulimab is an anti-programmed cell death-1 (PD-1) IgG1 antibody with no Fc gamma receptor (FcγR) binding activity, and thus theoretically reduced immune-related adverse events (irAEs) while maintaining efficacy. This single-arm, phase II trial conducted across 20 tertiary care centers in China enrolled adult patients with metastatic nasopharyngeal carcinoma (NPC) who had failed two or more lines of previous systemic chemotherapy. Patients received 200-mg penpulimab intravenously every 2 weeks (4 weeks per cycle) until disease progression or intolerable toxicities. The primary endpoint was objective response rate (ORR) per RECIST (version 1.1), as assessed by an independent radiological review committee. The secondary endpoints included progression-free survival (PFS) and overall survival (OS). One hundred thirty patients were enrolled and 125 were efficacy evaluable. At the data cutoff date (September 28, 2022), 1 patient achieved complete response and 34 patients attained partial response. The ORR was 28.0% (95% CI 20.3-36.7%). The response was durable, with 66.8% still in response at 9 months. Thirty-three patients (26.4%) were still on treatment. The median PFS and OS were 3.6 months (95% CI = 1.9-7.3 months) and 22.8 months (95% CI = 17.1 months to not reached), respectively. Ten (7.6%) patients experienced grade 3 or higher irAEs. Penpulimab has promising anti-tumor activities and acceptable toxicities in heavily pretreated metastatic NPC patients, supporting further clinical development as third-line treatment of metastatic NPC.
© 2024. The Author(s).
Conflict of interest statement
W.L., M.X., Y.X., Z.W., and B.L. are full-time employees of Akeso Biopharma, Inc. No competing interests were reported by any other contributing authors.
Figures
Similar articles
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
-
Adjuvant PD-1 Blockade With Camrelizumab for Nasopharyngeal Carcinoma: The DIPPER Randomized Clinical Trial.JAMA. 2025 May 13;333(18):1589-1598. doi: 10.1001/jama.2025.1132. JAMA. 2025. PMID: 40079940 Clinical Trial.
-
Zanidatamab plus chemotherapy as first-line treatment for patients with HER2-positive advanced gastro-oesophageal adenocarcinoma: primary results of a multicentre, single-arm, phase 2 study.Lancet Oncol. 2025 Jul;26(7):847-859. doi: 10.1016/S1470-2045(25)00287-6. Epub 2025 Jun 2. Lancet Oncol. 2025. PMID: 40473445 Clinical Trial.
-
Combined programmed cell death protein 1 and cytotoxic T-lymphocyte associated protein 4 blockade in an international cohort of patients with acral lentiginous melanoma.Br J Dermatol. 2025 Jan 24;192(2):316-326. doi: 10.1093/bjd/ljae401. Br J Dermatol. 2025. PMID: 39438074
-
Immunotherapy combined with chemotherapy without locoregional radiotherapy in de novo metastatic nasopharyngeal carcinoma: two case reports and a literature review.Front Immunol. 2025 Jan 9;15:1467355. doi: 10.3389/fimmu.2024.1467355. eCollection 2024. Front Immunol. 2025. PMID: 39850881 Free PMC article. Review.
Cited by
-
Efficacy and safety of first-line treatments for recurrent or metastatic nasopharyngeal carcinoma: a systematic review and network meta-analysis.Front Immunol. 2025 Jun 9;16:1485609. doi: 10.3389/fimmu.2025.1485609. eCollection 2025. Front Immunol. 2025. PMID: 40552283 Free PMC article.
-
The cost effectiveness of penpulimab with paclitaxel and carboplatin in first-line treatment of metastatic squamous non-small cell lung cancer.Sci Rep. 2025 Apr 12;15(1):12679. doi: 10.1038/s41598-025-97591-2. Sci Rep. 2025. PMID: 40221588 Free PMC article.
References
-
- Carioli, G., et al. Global trends in nasopharyngeal cancer mortality since 1970 and predictions for 2020: focus on low-risk areas. Int. J. Cancer140, 2256–2264 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

