Expression and functional implications of YME1L in nasopharyngeal carcinoma
- PMID: 38890304
- PMCID: PMC11189534
- DOI: 10.1038/s41419-024-06811-6
Expression and functional implications of YME1L in nasopharyngeal carcinoma
Abstract
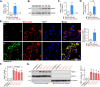
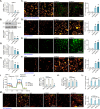
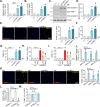
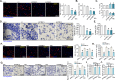
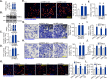
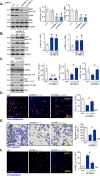
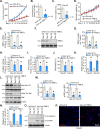
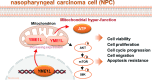
Mitochondria play a crucial role in the progression of nasopharyngeal carcinoma (NPC). YME1L, a member of the AAA ATPase family, is a key regulator of mitochondrial function and has been implicated in various cellular processes and diseases. This study investigates the expression and functional significance of YME1L in NPC. YME1L exhibits significant upregulation in NPC tissues from patients and across various primary human NPC cells, while its expression remains relatively low in adjacent normal tissues and primary nasal epithelial cells. Employing genetic silencing through the shRNA strategy or knockout (KO) via the CRISPR-sgRNA method, we demonstrated that YME1L depletion disrupted mitochondrial function, leading to mitochondrial depolarization, reactive oxygen species (ROS) generation, lipid peroxidation, and ATP reduction within primary NPC cells. Additionally, YME1L silencing or KO substantially impeded cell viability, proliferation, cell cycle progression, and migratory capabilities, concomitant with an augmentation of Caspase-apoptosis activation in primary NPC cells. Conversely, ectopic YME1L expression conferred pro-tumorigenic attributes, enhancing ATP production and bolstering NPC cell proliferation and migration. Moreover, our findings illuminate the pivotal role of YME1L in Akt-mTOR activation within NPC cells, with Akt-S6K phosphorylation exhibiting a significant decline upon YME1L depletion but enhancement upon YME1L overexpression. In YME1L-silenced primary NPC cells, the introduction of a constitutively-active Akt1 mutant (caAkt1, at S473D) restored Akt-S6K phosphorylation, effectively ameliorating the inhibitory effects imposed by YME1L shRNA. In vivo studies revealed that intratumoral administration of YME1L-shRNA-expressing adeno-associated virus (AAV) curtailed subcutaneous NPC xenograft growth in nude mice. Furthermore, YME1L downregulation, concurrent with mitochondrial dysfunction and ATP reduction, oxidative injury, Akt-mTOR inactivation, and apoptosis induction were evident within YME1L-silenced NPC xenograft tissues. Collectively, these findings shed light on the notable pro-tumorigenic role by overexpressed YME1L in NPC, with a plausible mechanism involving the promotion of Akt-mTOR activation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Targeting the mitochondrial protein YME1L to inhibit osteosarcoma cell growth in vitro and in vivo.Cell Death Dis. 2024 May 20;15(5):346. doi: 10.1038/s41419-024-06722-6. Cell Death Dis. 2024. PMID: 38769124 Free PMC article.
-
Overexpressed Gαi1 exerts pro-tumorigenic activity in nasopharyngeal carcinoma.Cell Death Dis. 2023 Dec 4;14(12):792. doi: 10.1038/s41419-023-06308-8. Cell Death Dis. 2023. PMID: 38049415 Free PMC article.
-
The requirement of the mitochondrial protein NDUFS8 for angiogenesis.Cell Death Dis. 2024 Apr 9;15(4):253. doi: 10.1038/s41419-024-06636-3. Cell Death Dis. 2024. PMID: 38594244 Free PMC article.
-
A systematic review of p53 regulation of oxidative stress in skeletal muscle.Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article.
-
Anti-ferroptosis: A Promising Therapeutic Approach in Nasopharyngeal Carcinoma.Front Biosci (Landmark Ed). 2025 Jun 20;30(6):27115. doi: 10.31083/FBL27115. Front Biosci (Landmark Ed). 2025. PMID: 40613277 Review.
Cited by
-
Targeting POLRMT by IMT1 inhibits colorectal cancer cell growth.Cell Death Dis. 2024 Sep 3;15(9):643. doi: 10.1038/s41419-024-07023-8. Cell Death Dis. 2024. PMID: 39227564 Free PMC article.
-
DIA/SWATH-Mass Spectrometry Revealing Melanoma Cell Proteome Transformations with Silver Nanoparticles: An Innovative Comparative Study.Int J Mol Sci. 2025 Feb 26;26(5):2029. doi: 10.3390/ijms26052029. Int J Mol Sci. 2025. PMID: 40076651 Free PMC article.
-
Mitochondrial carrier homolog 2 is important for mitochondrial functionality and non-small cell lung cancer cell growth.Cell Death Dis. 2025 Feb 13;16(1):95. doi: 10.1038/s41419-025-07419-0. Cell Death Dis. 2025. PMID: 39948081 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

