Augmented microglial endoplasmic reticulum-mitochondria contacts mediate depression-like behavior in mice induced by chronic social defeat stress
- PMID: 38890305
- PMCID: PMC11189428
- DOI: 10.1038/s41467-024-49597-z
Augmented microglial endoplasmic reticulum-mitochondria contacts mediate depression-like behavior in mice induced by chronic social defeat stress
Abstract
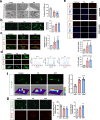
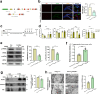
Extracellular ATP (eATP) signaling through the P2X7 receptor pathway is widely believed to trigger NLRP3 inflammasome assembly in microglia, potentially contributing to depression. However, the cellular stress responses of microglia to both eATP and stress itself remain largely unexplored. Mitochondria-associated membranes (MAMs) is a platform facilitating calcium transport between the endoplasmic reticulum (ER) and mitochondria, regulating ER stress responses and mitochondrial homeostasis. This study aims to investigate how MAMs influence microglial reaction and their involvement in the development of depression-like symptoms in response to chronic social defeat stress (CSDS). CSDS induced ER stress, MAMs' modifications, mitochondrial damage, and the formation of the IP3R3-GRP75-VDAC1 complex at the ER-mitochondria interface in hippocampal microglia, all concomitant with depression-like behaviors. Additionally, exposing microglia to eATP to mimic CSDS conditions resulted in analogous outcomes. Furthermore, knocking down GRP75 in BV2 cells impeded ER-mitochondria contact, calcium transfer, ER stress, mitochondrial damage, mitochondrial superoxide production, and NLRP3 inflammasome aggregation induced by eATP. In addition, reduced GRP75 expression in microglia of Cx3cr1CreER/+Hspa9f/+ mice lead to reduce depressive behaviors, decreased NLRP3 inflammasome aggregation, and fewer ER-mitochondria contacts in hippocampal microglia during CSDS. Here, we show the role of MAMs, particularly the formation of a tripartite complex involving IP3R3, GRP75, and VDAC1 within MAMs, in facilitating communication between the ER and mitochondria in microglia, thereby contributing to the development of depression-like phenotypes in male mice.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

