Protein restriction slows the development and progression of pathology in a mouse model of Alzheimer's disease
- PMID: 38890307
- PMCID: PMC11189507
- DOI: 10.1038/s41467-024-49589-z
Protein restriction slows the development and progression of pathology in a mouse model of Alzheimer's disease
Abstract
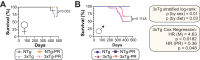
Dietary protein is a critical regulator of metabolic health and aging. Low protein diets are associated with healthy aging in humans, and dietary protein restriction extends the lifespan and healthspan of mice. In this study, we examined the effect of protein restriction (PR) on metabolic health and the development and progression of Alzheimer's disease (AD) in the 3xTg mouse model of AD. Here, we show that PR promotes leanness and glycemic control in 3xTg mice, specifically rescuing the glucose intolerance of 3xTg females. PR induces sex-specific alterations in circulating and brain metabolites, downregulating sphingolipid subclasses in 3xTg females. PR also reduces AD pathology and mTORC1 activity, increases autophagy, and improves the cognition of 3xTg mice. Finally, PR improves the survival of 3xTg mice. Our results suggest that PR or pharmaceutical interventions that mimic the effects of this diet may hold promise as a treatment for AD.
© 2024. The Author(s).
Conflict of interest statement
D.W.L. has received funding from, and is a scientific advisory board member of, Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases. J.M.D. is a consultant for Evrys Bio and co‐founder of Galilei BioSciences. The remaining authors declare no competing interests.
Figures







Update of
-
Protein restriction slows the development and progression of Alzheimer's disease in mice.Res Sq [Preprint]. 2024 Apr 24:rs.3.rs-3342413. doi: 10.21203/rs.3.rs-3342413/v2. Res Sq. 2024. Update in: Nat Commun. 2024 Jun 18;15(1):5217. doi: 10.1038/s41467-024-49589-z. PMID: 37790423 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- R56 AG056771/AG/NIA NIH HHS/United States
- F31 AG066311/AG/NIA NIH HHS/United States
- I01 BX004031/BX/BLRD VA/United States
- F31AG081115/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 AG084156/AG/NIA NIH HHS/United States
- R01 AG062328/AG/NIA NIH HHS/United States
- RF1 AG056771/AG/NIA NIH HHS/United States
- R35 GM149279/GM/NIGMS NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- T32 GM140935/GM/NIGMS NIH HHS/United States
- R01 AG078794/AG/NIA NIH HHS/United States
- U01 AG081482/AG/NIA NIH HHS/United States
- F31 AG081115/AG/NIA NIH HHS/United States
- R01 DK133479/DK/NIDDK NIH HHS/United States
- IS1 BX005524/BX/BLRD VA/United States
- R01 AG056771/AG/NIA NIH HHS/United States
- F31 AG082504/AG/NIA NIH HHS/United States
- R35 GM151130/GM/NIGMS NIH HHS/United States
- 23AARG-1029665/Alzheimer's Association
- AG082504/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R21 AG061635/AG/NIA NIH HHS/United States
- R35GM151130/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- F32 AG077916/AG/NIA NIH HHS/United States
- R01DK133479/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- T32 AG000213/AG/NIA NIH HHS/United States
- R01 GM148487/GM/NIGMS NIH HHS/United States
- R01 NS094154/NS/NINDS NIH HHS/United States
- AG056771, AG062328, AG061635, AG081482, AG084156/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 DK125859/DK/NIDDK NIH HHS/United States
- K12 HD101368/HD/NICHD NIH HHS/United States
- DK125859/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

