Regulation of cellular and systemic sphingolipid homeostasis
- PMID: 38890457
- PMCID: PMC12034107
- DOI: 10.1038/s41580-024-00742-y
Regulation of cellular and systemic sphingolipid homeostasis
Abstract
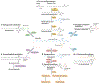
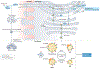
One hundred and fifty years ago, Johann Thudichum described sphingolipids as unusual "Sphinx-like" lipids from the brain. Today, we know that thousands of sphingolipid molecules mediate many essential functions in embryonic development and normal physiology. In addition, sphingolipid metabolism and signalling pathways are dysregulated in a wide range of pathologies, and therapeutic agents that target sphingolipids are now used to treat several human diseases. However, our understanding of sphingolipid regulation at cellular and organismal levels and their functions in developmental, physiological and pathological settings is rudimentary. In this Review, we discuss recent advances in sphingolipid pathways in different organelles, how secreted sphingolipid mediators modulate physiology and disease, progress in sphingolipid-targeted therapeutic and diagnostic research, and the trans-cellular sphingolipid metabolic networks between microbiota and mammals. Advances in sphingolipid biology have led to a deeper understanding of mammalian physiology and may lead to progress in the management of many diseases.
© 2024. Springer Nature Limited.
Figures


References
-
- Thudichum JLW A treatise on the chemical constitution of the brain. (Archon Books, 1962).
-
- Carter HE & Humiston CG Biochemistry of the sphingolipides. V. The structure of sphingine. J Biol Chem 191, 727–733 (1951). - PubMed
-
- Klenk E [Contribution to the concept of gangliosides]. Hoppe Seylers Z Physiol Chem 288, 216–220 (1951). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

