Membrane depolarization mediates both the inhibition of neural activity and cell-type-differences in response to high-frequency stimulation
- PMID: 38890481
- PMCID: PMC11189419
- DOI: 10.1038/s42003-024-06359-3
Membrane depolarization mediates both the inhibition of neural activity and cell-type-differences in response to high-frequency stimulation
Abstract
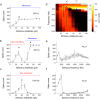
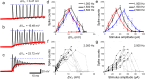
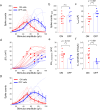
Neuromodulation using high frequency (>1 kHz) electric stimulation (HFS) enables preferential activation or inhibition of individual neural types, offering the possibility of more effective treatments across a broad spectrum of neurological diseases. To improve effectiveness, it is important to better understand the mechanisms governing activation and inhibition with HFS so that selectivity can be optimized. In this study, we measure the membrane potential (Vm) and spiking responses of ON and OFF α-sustained retinal ganglion cells (RGCs) to a wide range of stimulus frequencies (100-2500 Hz) and amplitudes (10-100 µA). Our findings indicate that HFS induces shifts in Vm, with both the strength and polarity of the shifts dependent on the stimulus conditions. Spiking responses in each cell directly correlate with the shifts in Vm, where strong depolarization leads to spiking suppression. Comparisons between the two cell types reveal that ON cells are more depolarized by a given amplitude of HFS than OFF cells-this sensitivity difference enables the selective targeting. Computational modeling indicates that ion-channel dynamics largely account for the shifts in Vm, suggesting that a better understanding of the differences in ion-channel properties across cell types may improve the selectivity and ultimately, enhance HFS-based neurostimulation strategies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Grants and funding
- VR170089/United States Department of Defense | United States Army | Army Medical Command | Congressionally Directed Medical Research Programs (CDMRP)
- P 35488/FWF_/Austrian Science Fund FWF/Austria
- FWF P35488/Austrian Science Fund (Fonds zur Förderung der Wissenschaftlichen Forschung)
- 2022M3E5E8017395/National Research Foundation of Korea (NRF)
- R01 NS110575/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

