Decoding the hallmarks of allograft dysfunction with a comprehensive pan-organ transcriptomic atlas
- PMID: 38890530
- PMCID: PMC11645273
- DOI: 10.1038/s41591-024-03030-6
Decoding the hallmarks of allograft dysfunction with a comprehensive pan-organ transcriptomic atlas
Abstract
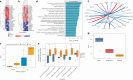
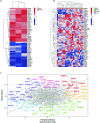
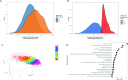
The pathogenesis of allograft (dys)function has been increasingly studied using 'omics'-based technologies, but the focus on individual organs has created knowledge gaps that neither unify nor distinguish pathological mechanisms across allografts. Here we present a comprehensive study of human pan-organ allograft dysfunction, analyzing 150 datasets with more than 12,000 samples across four commonly transplanted solid organs (heart, lung, liver and kidney, n = 1,160, 1,241, 1,216 and 8,853 samples, respectively) that we leveraged to explore transcriptomic differences among allograft dysfunction (delayed graft function, acute rejection and fibrosis), tolerance and stable graft function. We identified genes that correlated robustly with allograft dysfunction across heart, lung, liver and kidney transplantation. Furthermore, we developed a transfer learning omics prediction framework that, by borrowing information across organs, demonstrated superior classifications compared to models trained on single organs. These findings were validated using a single-center prospective kidney transplant cohort study (a collective 329 samples across two timepoints), providing insights supporting the potential clinical utility of our approach. Our study establishes the capacity for machine learning models to learn across organs and presents a transcriptomic transplant resource that can be employed to develop pan-organ biomarkers of allograft dysfunction.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: P.J.O. is a consultant for VericiDx and Qihan Biotech. H.R., J.Y.H.Y., N.M.R. and E.P. are inventors on two pending patent applications related to the research presented in this manuscript. These applications are currently under review for novelty and, if granted, could potentially influence the interpretation of the research findings. The authors declare that this does not alter their adherence to all the Nature Medicine policies on sharing data and materials, as detailed in the guidelines for authors. All other authors declare no competing interests.
Figures









References
-
- Wolfe, R. A. et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med.341, 1725–1730 (1999). - PubMed
-
- Rana, A. et al. Survival benefit of solid-organ transplant in the United States. JAMA Surg.150, 252–259 (2015). - PubMed
-
- Tonelli, M. et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am. J. Transpl.11, 2093–2109 (2011). - PubMed
-
- ANZDATA Registry. 43rd Report, Chapter 7: Kidney Transplantation. (Australia and New Zealand Dialysis and Transplant Registry, 2020); https://www.anzdata.org.au/wp-content/uploads/2020/09/c07_transplant_201...
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

