Comprehensive network of stress-induced responses in Zymomonas mobilis during bioethanol production: from physiological and molecular responses to the effects of system metabolic engineering
- PMID: 38890644
- PMCID: PMC11186258
- DOI: 10.1186/s12934-024-02459-1
Comprehensive network of stress-induced responses in Zymomonas mobilis during bioethanol production: from physiological and molecular responses to the effects of system metabolic engineering
Abstract
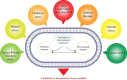
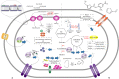
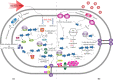
Nowadays, biofuels, especially bioethanol, are becoming increasingly popular as an alternative to fossil fuels. Zymomonas mobilis is a desirable species for bioethanol production due to its unique characteristics, such as low biomass production and high-rate glucose metabolism. However, several factors can interfere with the fermentation process and hinder microbial activity, including lignocellulosic hydrolysate inhibitors, high temperatures, an osmotic environment, and high ethanol concentration. Overcoming these limitations is critical for effective bioethanol production. In this review, the stress response mechanisms of Z. mobilis are discussed in comparison to other ethanol-producing microbes. The mechanism of stress response is divided into physiological (changes in growth, metabolism, intracellular components, and cell membrane structures) and molecular (up and down-regulation of specific genes and elements of the regulatory system and their role in expression of specific proteins and control of metabolic fluxes) changes. Systemic metabolic engineering approaches, such as gene manipulation, overexpression, and silencing, are successful methods for building new metabolic pathways. Therefore, this review discusses systems metabolic engineering in conjunction with systems biology and synthetic biology as an important method for developing new strains with an effective response mechanism to fermentation stresses during bioethanol production. Overall, understanding the stress response mechanisms of Z. mobilis can lead to more efficient and effective bioethanol production.
Keywords: Zymomonas mobilis; Bioethanol fermentation stress condition; Metabolic engineering; Stress response regulatory network; Synthetic biology; Systems biology.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
New technologies provide more metabolic engineering strategies for bioethanol production in Zymomonas mobilis.Appl Microbiol Biotechnol. 2019 Mar;103(5):2087-2099. doi: 10.1007/s00253-019-09620-6. Epub 2019 Jan 19. Appl Microbiol Biotechnol. 2019. PMID: 30661108 Review.
-
Perspectives and new directions for bioprocess optimization using Zymomonas mobilis in the ethanol production.World J Microbiol Biotechnol. 2020 Jul 13;36(8):112. doi: 10.1007/s11274-020-02885-4. World J Microbiol Biotechnol. 2020. PMID: 32656581 Review.
-
Advances and prospects in metabolic engineering of Zymomonas mobilis.Metab Eng. 2018 Nov;50:57-73. doi: 10.1016/j.ymben.2018.04.001. Epub 2018 Apr 5. Metab Eng. 2018. PMID: 29627506 Review.
-
Adaptive Laboratory Evolution and Metabolic Engineering of Zymomonas mobilis for Bioethanol Production Using Molasses.ACS Synth Biol. 2023 Apr 21;12(4):1297-1307. doi: 10.1021/acssynbio.3c00056. Epub 2023 Apr 10. ACS Synth Biol. 2023. PMID: 37036829
-
Inhibition analysis of inhibitors derived from lignocellulose pretreatment on the metabolic activity of Zymomonas mobilis biofilm and planktonic cells and the proteomic responses.Biotechnol Bioeng. 2018 Jan;115(1):70-81. doi: 10.1002/bit.26449. Epub 2017 Oct 23. Biotechnol Bioeng. 2018. PMID: 28892134
Cited by
-
Altered sterol composition mediates multiple tolerance of Kluyveromyces marxianus for xylitol production.Microb Cell Fact. 2024 Oct 10;23(1):271. doi: 10.1186/s12934-024-02546-3. Microb Cell Fact. 2024. PMID: 39385269 Free PMC article.
References
-
- Ajit A, Sulaiman AZ, Chisti Y. Production of bioethanol by Zymomonas mobilis in high-gravity extractive fermentations. Food Bioprod Process. 2017;102:123–35. doi: 10.1016/j.fbp.2016.12.006. - DOI
-
- Khambhaty Y, Upadhyay D, Kriplani Y, Joshi N, Mody K, Gandhi MR. Bioethanol from Macroalgal Biomass: utilization of Marine yeast for production of the same. Bioenergy Res. 2013;6:188–95. doi: 10.1007/s12155-012-9249-4. - DOI
-
- Hu N, Yuan B, Sun J, Wang S-A, Li F-L. Thermotolerant Kluyveromyces marxianus and Saccharomyces cerevisiae strains representing potentials for bioethanol production from Jerusalem artichoke by consolidated bioprocessing. Appl Microbiol Biotechnol. 2012;95:1359–68. doi: 10.1007/s00253-012-4240-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

