Clinical, imaging and histopathological characterization of a series of three cats with cerebellar cortical degeneration
- PMID: 38890680
- PMCID: PMC11186075
- DOI: 10.1186/s12917-024-04127-3
Clinical, imaging and histopathological characterization of a series of three cats with cerebellar cortical degeneration
Abstract
Background: Neurological inherited disorders are rare in domestic animals. Cerebellar cortical degeneration remains amongst the most common of these disorders. The condition is defined as the premature loss of fully differentiated cerebellar components due to genetic or metabolic defects. It has been studied in dogs and cats, and various genetic defects and diagnostic tests (including magnetic resonance imaging (MRI)) have been refined in these species. Cases in cats remain rare and mostly individual, and few diagnostic criteria, other than post-mortem exam, have been evaluated in reports with multiple cases. Here, we report three feline cases of cerebellar cortical degeneration with detailed clinical, diagnostic imaging and post-mortem findings.
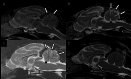
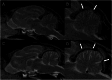
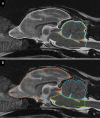
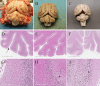
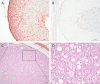
Case presentation: The three cases were directly (siblings, case #1 and #2) or indirectly related (same farm, case #3) and showed early-onset of the disease, with clinical signs including cerebellar ataxia and tremors. Brain MRI was highly suggestive of cerebellar cortical degeneration on all three cases. The relative cerebrospinal fluid (CSF) space, relative cerebellum size, brainstem: cerebellum area ratio, and cerebellum: total brain area ratio, were measured and compared to a control group of cats and reference cut-offs for dogs in the literature. For the relative cerebellum size and cerebellum: total brain area ratio, all affected cases had a lower value than the control group. For the relative CSF space and brainstem: cerebellum area ratio, the more affected cases (#2 and #3) had higher values than the control group, while the least affected case (#3) had values within the ranges of the control group, but a progression was visible over time. Post-mortem examination confirmed the diagnosis of cerebellar cortical degeneration, with marked to complete loss of Purkinje cells and associated granular layer depletion and proliferation of Bergmann glia. One case also had Wallerian-like degeneration in the spinal cord, suggestive of spinocerebellar degeneration.
Conclusion: Our report further supports a potential genetic component for the disease in cats. For the MRI examination, the relative cerebellum size and cerebellum: total brain area ratio seem promising, but further studies are needed to establish specific feline cut-offs. Post-mortem evaluation of the cerebellum remains the gold standard for the final diagnosis.
Keywords: Cerebellar cortical degeneration; Genetic; Inherited; MRI; Purkinje cells.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Computer-assisted magnetic resonance imaging brain morphometry in American Staffordshire Terriers with cerebellar cortical degeneration.J Vet Intern Med. 2008 Jul-Aug;22(4):969-75. doi: 10.1111/j.1939-1676.2008.0138.x. J Vet Intern Med. 2008. PMID: 18647158
-
Histopathological and ultrastructural features of feline hereditary cerebellar cortical atrophy: a novel animal model of human spinocerebellar degeneration.Acta Neuropathol. 1998 Oct;96(4):379-87. doi: 10.1007/s004010050908. Acta Neuropathol. 1998. PMID: 9797002
-
Late onset cerebellar degeneration in a middle-aged cat.J Feline Med Surg. 2006 Dec;8(6):424-9. doi: 10.1016/j.jfms.2006.04.007. Epub 2006 Jun 15. J Feline Med Surg. 2006. PMID: 16781880 Free PMC article.
-
Primary degeneration of the granular layer of the cerebellum. A study of 14 patients and review of the literature.Neuropediatrics. 1994 Aug;25(4):183-90. doi: 10.1055/s-2008-1073020. Neuropediatrics. 1994. PMID: 7824090 Review.
-
MRI characteristics of suspected acute spinal cord infarction in two cats, and a review of the literature.J Feline Med Surg. 2005 Apr;7(2):101-7. doi: 10.1016/j.jfms.2004.08.001. J Feline Med Surg. 2005. PMID: 15771946 Free PMC article. Review.
Cited by
-
Exosomes in Regulating miRNAs for Biomarkers of Neurodegenerative Disorders.Mol Neurobiol. 2025 Jun;62(6):7576-7596. doi: 10.1007/s12035-025-04733-8. Epub 2025 Feb 7. Mol Neurobiol. 2025. PMID: 39918711 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

