Sexually dimorphic effects of prenatal alcohol exposure on the murine skeleton
- PMID: 38890762
- PMCID: PMC11186175
- DOI: 10.1186/s13293-024-00626-y
Sexually dimorphic effects of prenatal alcohol exposure on the murine skeleton
Abstract
Background: Prenatal alcohol exposure (PAE) can result in lifelong disabilities known as foetal alcohol spectrum disorder (FASD) and is associated with childhood growth deficiencies and increased bone fracture risk. However, the effects of PAE on the adult skeleton remain unclear and any potential sexual dimorphism is undetermined. Therefore, we utilised a murine model to examine sex differences with PAE on in vitro bone formation, and in the juvenile and adult skeleton.
Methods: Pregnant C57BL/6J female mice received 5% ethanol in their drinking water during gestation. Primary calvarial osteoblasts were isolated from neonatal offspring and mineralised bone nodule formation and gene expression assessed. Skeletal phenotyping of 4- and 12-week-old male and female offspring was conducted by micro-computed tomography (µCT), 3-point bending, growth plate analyses, and histology.
Results: Osteoblasts from male and female PAE mice displayed reduced bone formation, compared to control (≤ 30%). Vegfa, Vegfb, Bmp6, Tgfbr1, Flt1 and Ahsg were downregulated in PAE male osteoblasts only, whilst Ahsg was upregulated in PAE females. In 12-week-old mice, µCT analysis revealed a sex and exposure interaction across several trabecular bone parameters. PAE was detrimental to the trabecular compartment in male mice compared to control, yet PAE females were unaffected. Both male and female mice had significant reductions in cortical parameters with PAE. Whilst male mice were negatively affected along the tibial length, females were only distally affected. Posterior cortical porosity was increased in PAE females only. Mechanical testing revealed PAE males had significantly reduced bone stiffness compared to controls; maximum load and yield were reduced in both sexes. PAE had no effect on total body weight or tibial bone length in either sex. However, total growth plate width in male PAE mice compared to control was reduced, whilst female PAE mice were unaffected. 4-week-old mice did not display the altered skeletal phenotype with PAE observed in 12-week-old animals.
Conclusions: Evidence herein suggests, for the first time, that PAE exerts divergent sex effects on the skeleton, possibly influenced by underlying sex-specific transcriptional mechanisms of osteoblasts. Establishing these sex differences will support future policies and clinical management of FASD.
Keywords: Bone; Murine model; Prenatal alcohol exposure (PAE); Sexual dimorphism.
Plain language summary
Prenatal alcohol exposure (PAE) can lead to a set of lifelong cognitive, behavioural, and physical disabilities known as foetal alcohol spectrum disorder (FASD). FASD is a significant burden on healthcare, justice and education systems, which is set to worsen with rising alcohol consumption rates. FASD children have an increased risk of long bone fracture and adolescents are smaller in stature. However, sex differences and the long-term effects of PAE on the skeleton have not been investigated and was the aim of this study. Using a mouse model of PAE, we examined the function and gene expression of bone-forming cells (osteoblasts). We then analysed the skeletons of male and female mice at 12-weeks-old (adult) and 4-weeks-old (juvenile). PAE reduced osteoblast bone formation in both sexes, compared to control. Differential gene expression was predominantly observed in PAE males and largely involved genes related to blood vessel formation. High resolution x-ray imaging (micro-CT) revealed PAE had a detrimental effect on the inner trabecular bone component in 12-week-old male mice only. Analysis of the outer cortical bone revealed that whilst both male and female PAE mice were negatively affected, anatomical variations were observed. Mechanical testing also revealed differences in bone strength in PAE mice, compared to control. Interestingly, 4-week-old mice did not possess these sex differences observed in our PAE model at 12 weeks of age. Our data suggest PAE has detrimental and yet sex-dependent effects on the skeleton. Establishing these sex differences will support future policies and clinical management of FASD.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


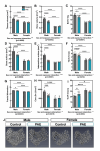
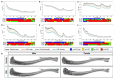




Similar articles
-
Sex-specific deficits in biochemical but not behavioral responses to delay fear conditioning in prenatal alcohol exposure mice.Neurobiol Learn Mem. 2018 Dec;156:1-16. doi: 10.1016/j.nlm.2018.10.002. Epub 2018 Oct 12. Neurobiol Learn Mem. 2018. PMID: 30316893 Free PMC article.
-
Does prenatal alcohol exposure cause a metabolic syndrome? (Non-)evidence from a mouse model of fetal alcohol spectrum disorder.PLoS One. 2018 Jun 28;13(6):e0199213. doi: 10.1371/journal.pone.0199213. eCollection 2018. PLoS One. 2018. PMID: 29953483 Free PMC article.
-
Prenatal alcohol exposure programmes offspring disease: insulin resistance in adult males in a rat model of acute exposure.J Physiol. 2019 Dec;597(23):5619-5637. doi: 10.1113/JP278531. Epub 2019 Nov 13. J Physiol. 2019. PMID: 31595508
-
Effects of prenatal alcohol exposure (PAE): insights into FASD using mouse models of PAE.Biochem Cell Biol. 2018 Apr;96(2):131-147. doi: 10.1139/bcb-2017-0280. Epub 2018 Jan 25. Biochem Cell Biol. 2018. PMID: 29370535 Free PMC article. Review.
-
Prenatal alcohol exposure and associations with physical size, dysmorphology and neurodevelopment: a systematic review and meta-analysis.BMC Med. 2024 Oct 15;22(1):467. doi: 10.1186/s12916-024-03656-w. BMC Med. 2024. PMID: 39407296 Free PMC article.
References
-
- Javaid MK, Cooper C. Prenatal and childhood influences on osteoporosis. Best Pract Res Clin Endocrinol Metab. 2002;16(2):349 – 67. 10.1053/beem.2002.0199. PubMed PMID: 12064897. - PubMed
-
- Jensen KH, Riis KR, Abrahamsen B, Handel MN. Nutrients, Diet, and Other Factors in Prenatal Life and Bone Health in Young Adults: A Systematic Review of Longitudinal Studies. Nutrients. 2020;12(9). Epub 20200919. 10.3390/nu12092866. PubMed PMID: 32961712; PubMed Central PMCID: PMCPMC7551661. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- MR/R022240/2/MRC_/Medical Research Council/United Kingdom
- MR/V033506/1/MRC_/Medical Research Council/United Kingdom
- BBS/E/D/10002071 and BBS/E/RL/230001C/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/V033506/1 & MR/R022240/2/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous

