The TF/Nrf2/GSTP1 pathway is involved in stress-induced hepatocellular injury through ferroptosis
- PMID: 38890797
- PMCID: PMC11187937
- DOI: 10.1111/jcmm.18494
The TF/Nrf2/GSTP1 pathway is involved in stress-induced hepatocellular injury through ferroptosis
Abstract
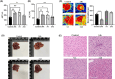
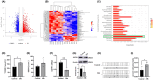
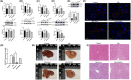
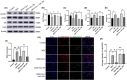
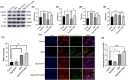
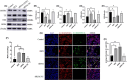
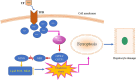
Stress triggers a comprehensive pathophysiological cascade in organisms. However, there is a substantial gap in the research regarding the effects of stress on liver function. This study aimed to investigate the impact of restraint stress on hepatocellular damage and elucidate the underlying molecular mechanisms. An effective mouse restraint stress model was successfully developed, and liver function analysis was performed using laser speckle imaging, metabolomics and serum testing. Alterations in hepatocyte morphology were assessed using haematoxylin and eosin staining and transmission electron microscopy. Oxidative stress in hepatocytes was assessed using lipid reactive oxygen species and malondialdehyde. The methylation status and expression of GSTP1 were analysed using DNA sequencing and, real-time PCR, and the expression levels of GPX4, TF and Nrf2 were evaluated using real-time quantitative PCR, western blotting, and immunohistochemical staining. A stress-induced model was established in vitro by using dexamethasone-treated AML-12 cells. To investigate the underlying mechanisms, GSTP1 overexpression, small interfering RNA, ferroptosis and Nrf2 inhibitors were used. GSTP1 methylation contributes to stress-induced hepatocellular damage and dysfunction. GSTP1 is involved in ferroptosis-mediated hepatocellular injury induced by restraint stress via the TF/Nrf2 pathway. These findings suggest that stress-induced hepatocellular injury is associated with ferroptosis, which is regulated by TF/Nrf2/GSTP1.
Keywords: TF/Nrf2/GSTP1; ferroptosis; liver injury; restraint stress.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Daolin T. Ferroptosis in Health and Disease; Springer Cham, 2019:324.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

