Sorghum Grain Polyphenolic Extracts Demonstrate Neuroprotective Effects Related to Alzheimer's Disease in Cellular Assays
- PMID: 38890943
- PMCID: PMC11171927
- DOI: 10.3390/foods13111716
Sorghum Grain Polyphenolic Extracts Demonstrate Neuroprotective Effects Related to Alzheimer's Disease in Cellular Assays
Abstract
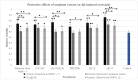
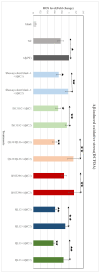
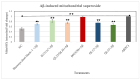
Sorghum grain contains high levels and a diverse profile of polyphenols (PPs), which are antioxidants known to reduce oxidative stress when consumed in the diet. Oxidative stress leading to amyloid-β (Aβ) aggregation, neurotoxicity, and mitochondrial dysfunction is implicated in the pathogenesis of Alzheimer's disease (AD). Thus, PPs have gained attention as possible therapeutic agents for combating AD. This study aimed to (a) quantify the phenolic compounds (PP) and antioxidant capacities in extracts from six different varieties of sorghum grain and (b) investigate whether these PP extracts exhibit any protective effects on human neuroblastoma (BE(2)-M17) cells against Aβ- and tau-induced toxicity, Aβ aggregation, mitochondrial dysfunction, and reactive oxygen species (ROS) induced by Aβ and tert-butyl hydroperoxide (TBHP). PP and antioxidant capacity were quantified using chemical assays. Aβ- and tau-induced toxicity was determined using the 3-(4,5-dimenthylthiazol-2-yl)-2,5-dimethyltetrazolium bromide (MTS) assay. The thioflavin T (Th-T) assay assessed anti-Aβ aggregation. The dichlorodihydrofluorescein diacetate (DCFDA) assay determined the levels of general ROS and the MitoSOX assay determined the levels of mitochondrial superoxide. Sorghum varieties Shawaya short black-1 and IS1311C possessed the highest levels of total phenolics, total flavonoids, and antioxidant capacity, and sorghum varieties differed significantly in their profile of individual PPs. All extracts significantly increased cell viability compared to the control (minus extract). Variety QL33 (at 2000 µg sorghum flour equivalents/mL) showed the strongest protective effect with a 28% reduction in Aβ-toxicity cell death. The extracts of all sorghum varieties significantly reduced Aβ aggregation. All extracts except that from variety B923296 demonstrated a significant (p ≤ 0.05) downregulation of Aβ-induced and TBHP-induced ROS and mitochondrial superoxide relative to the control (minus extract) in a dose- and variety-dependent manner. We have demonstrated for the first time that sorghum polyphenolic extracts show promising neuroprotective effects against AD, which indicates the potential of sorghum foods to exert a similar beneficial property in the human diet. However, further analysis in other cellular models and in vivo is needed to confirm these effects.
Keywords: Alzheimer’s disease; amyloid-β; antioxidants; mitochondrial function; neuroprotection; polyphenols; reactive oxygen species; sorghum.
Conflict of interest statement
Edith Cowan University staff members and Cwek Pty were involved in the design of the study; in the collection, analyses, or interpretation of data; and in the writing of the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Investigating the Impact of Sorghum on Tau Protein Phosphorylation and Mitochondrial Dysfunction Modulation in Alzheimer's Disease: An In Vitro Study.Nutrients. 2025 Jan 30;17(3):516. doi: 10.3390/nu17030516. Nutrients. 2025. PMID: 39940374 Free PMC article.
-
Potential of Sorghum Polyphenols to Prevent and Treat Alzheimer's Disease: A Review Article.Front Aging Neurosci. 2021 Oct 6;13:729949. doi: 10.3389/fnagi.2021.729949. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34690742 Free PMC article. Review.
-
Greek Sage Exhibits Neuroprotective Activity against Amyloid Beta-Induced Toxicity.Evid Based Complement Alternat Med. 2020 Dec 7;2020:2975284. doi: 10.1155/2020/2975284. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33505483 Free PMC article.
-
Piper sarmentosum Roxb. Attenuates Beta Amyloid (Aβ)-Induced Neurotoxicity Via the Inhibition of Amyloidogenesis and Tau Hyperphosphorylation in SH-SY5Y Cells.Curr Alzheimer Res. 2021;18(1):80-87. doi: 10.2174/1567205018666210324124239. Curr Alzheimer Res. 2021. PMID: 33761853
-
Mechanistic Insight into the Design of Chemical Tools to Control Multiple Pathogenic Features in Alzheimer's Disease.Acc Chem Res. 2021 Oct 19;54(20):3930-3940. doi: 10.1021/acs.accounts.1c00457. Epub 2021 Oct 4. Acc Chem Res. 2021. PMID: 34606227 Review.
Cited by
-
Investigating the in-vitro antimicrobial activities of sorghum [Sorghum bicolor (L.) Moench] phenolic extracts on liver abscess causing bacterial pathogens.Front Cell Infect Microbiol. 2025 Jun 26;15:1568504. doi: 10.3389/fcimb.2025.1568504. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40642104 Free PMC article.
-
Experimental Evidence of Caffeic Acid's Neuroprotective Activity in Alzheimer's Disease: In Vitro, In Vivo, and Delivery-Based Insights.Medicina (Kaunas). 2025 Aug 8;61(8):1428. doi: 10.3390/medicina61081428. Medicina (Kaunas). 2025. PMID: 40870473 Free PMC article.
-
Investigating the Impact of Sorghum on Tau Protein Phosphorylation and Mitochondrial Dysfunction Modulation in Alzheimer's Disease: An In Vitro Study.Nutrients. 2025 Jan 30;17(3):516. doi: 10.3390/nu17030516. Nutrients. 2025. PMID: 39940374 Free PMC article.
References
-
- Stefoska-Needham A., Beck E.J., Johnson S.K., Tapsell L.C. Sorghum: An underutilized cereal whole grain with the potential to assist in the prevention of chronic disease. Food Rev. Internat. 2015;31:401–437. doi: 10.1080/87559129.2015.1022832. - DOI
-
- Licata R., Chu J., Wang S., Coorey R., James A., Zhao Y., Johnson S. Determination of formulation and processing factors affecting slowly digestible starch, protein digestibility and antioxidant capacity of extruded sorghum–maize composite flour. Int. J. Food Sci. Technol. 2014;49:1408–1419. doi: 10.1111/ijfs.12444. - DOI
-
- Kangama C.O. Importance of Sorghum bicolor in African’s cultures. J. Agric. Enviro. Sci. 2017;6:134–137. doi: 10.15640/jaes.v6n2a16. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

