The Effects of Smoking on Telomere Length, Induction of Oncogenic Stress, and Chronic Inflammatory Responses Leading to Aging
- PMID: 38891017
- PMCID: PMC11172003
- DOI: 10.3390/cells13110884
The Effects of Smoking on Telomere Length, Induction of Oncogenic Stress, and Chronic Inflammatory Responses Leading to Aging
Abstract
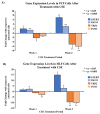
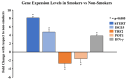
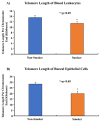
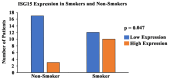
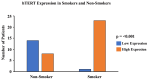
Telomeres, potential biomarkers of aging, are known to shorten with continued cigarette smoke exposure. In order to further investigate this process and its impact on cellular stress and inflammation, we used an in vitro model with cigarette smoke extract (CSE) and observed the downregulation of telomere stabilizing TRF2 and POT1 genes after CSE treatment. hTERT is a subunit of telomerase and a well-known oncogenic marker, which is overexpressed in over 85% of cancers and may contribute to lung cancer development in smokers. We also observed an increase in hTERT and ISG15 expression levels after CSE treatment, as well as increased protein levels revealed by immunohistochemical staining in smokers' lung tissue samples compared to non-smokers. The effects of ISG15 overexpression were further studied by quantifying IFN-γ, an inflammatory protein induced by ISG15, which showed greater upregulation in smokers compared to non-smokers. Similar changes in gene expression patterns for TRF2, POT1, hTERT, and ISG15 were observed in blood and buccal swab samples from smokers compared to non-smokers. The results from this study provide insight into the mechanisms behind smoking causing telomere shortening and how this may contribute to the induction of inflammation and/or tumorigenesis, which may lead to comorbidities in smokers.
Keywords: IFN-γ; ISG15; POT1; TRF2; aging; hTERT; smokers; telomere position effect; telomeres.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

