Plasma Gelsolin Inhibits Natural Killer Cell Function and Confers Chemoresistance in Epithelial Ovarian Cancer
- PMID: 38891037
- PMCID: PMC11171658
- DOI: 10.3390/cells13110905
Plasma Gelsolin Inhibits Natural Killer Cell Function and Confers Chemoresistance in Epithelial Ovarian Cancer
Abstract
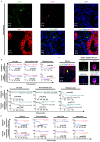
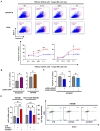
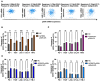
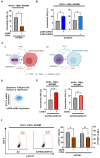
Plasma gelsolin (pGSN) overexpression in ovarian cancer (OVCA) disarms immune function, contributing to chemoresistance. The aim of this study was to investigate the immunoregulatory effects of pGSN expression on natural killer (NK) cell function in OVCA. OVCA tissues from primary surgeries underwent immunofluorescent staining of pGSN and the activated NK cell marker natural cytotoxicity triggering receptor 1 to analyze the prognostic impact of pGSN expression and activated NK cell infiltration. The immunoregulatory effects of pGSN on NK cells were assessed using apoptosis assay, cytokine secretion, immune checkpoint-receptor expression, and phosphorylation of STAT3. In OVCA tissue analyses, activated NK cell infiltration provided survival advantages to patients. However, high pGSN expression attenuated the survival benefits of activated NK cell infiltration. In the in vitro experiment, pGSN in OVCA cells induced NK cell death through cell-to-cell contact. pGSN increased T-cell immunoglobulin and mucin-domain-containing-3 expression (TIM-3) on activated NK cells. Further, it decreased interferon-γ production in activated TIM-3+ NK cells, attenuating their anti-tumor effects. Thus, increased pGSN expression suppresses the anti-tumor functions of NK cells. The study provides insights into why immunotherapy is rarely effective in patients with OVCA and suggests novel treatment strategies.
Keywords: chemoresistance; chemotherapy; gynecological cancers; natural killer cells; ovarian cancer; plasma gelsolin; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Exosomal Plasma Gelsolin Is an Immunosuppressive Mediator in the Ovarian Tumor Microenvironment and a Determinant of Chemoresistance.Cells. 2022 Oct 20;11(20):3305. doi: 10.3390/cells11203305. Cells. 2022. PMID: 36291171 Free PMC article. Review.
-
Plasma Gelsolin Inhibits CD8+ T-cell Function and Regulates Glutathione Production to Confer Chemoresistance in Ovarian Cancer.Cancer Res. 2020 Sep 15;80(18):3959-3971. doi: 10.1158/0008-5472.CAN-20-0788. Epub 2020 Jul 8. Cancer Res. 2020. PMID: 32641415
-
The regulation of plasma gelsolin by DNA methylation in ovarian cancer chemo-resistance.J Ovarian Res. 2024 Jan 12;17(1):15. doi: 10.1186/s13048-023-01332-w. J Ovarian Res. 2024. PMID: 38216951 Free PMC article.
-
Plasma Gelsolin Confers Chemoresistance in Ovarian Cancer by Resetting the Relative Abundance and Function of Macrophage Subtypes.Cancers (Basel). 2022 Feb 18;14(4):1039. doi: 10.3390/cancers14041039. Cancers (Basel). 2022. PMID: 35205790 Free PMC article.
-
NK cell-based therapeutics for lung cancer.Expert Opin Biol Ther. 2020 Jan;20(1):23-33. doi: 10.1080/14712598.2020.1688298. Epub 2019 Nov 12. Expert Opin Biol Ther. 2020. PMID: 31714156 Review.
Cited by
-
Gelsolin (GSN) as a key regulator in estrogen receptor-positive breast cancer: implications for prognosis, chemotherapy sensitivity, and immune infiltration.Discov Oncol. 2025 Jul 9;16(1):1294. doi: 10.1007/s12672-025-03128-4. Discov Oncol. 2025. PMID: 40634593 Free PMC article.
-
Spotlight on nuclear PD-L1 in ovarian cancer chemoresistance: hidden but mighty.Front Immunol. 2025 Jul 14;16:1543529. doi: 10.3389/fimmu.2025.1543529. eCollection 2025. Front Immunol. 2025. PMID: 40726982 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

