Adipo-Epithelial Transdifferentiation in In Vitro Models of the Mammary Gland
- PMID: 38891075
- PMCID: PMC11171678
- DOI: 10.3390/cells13110943
Adipo-Epithelial Transdifferentiation in In Vitro Models of the Mammary Gland
Abstract
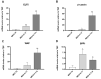
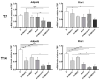
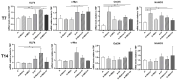
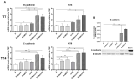
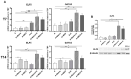
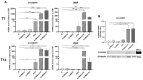
Subcutaneous adipocytes are crucial for mammary gland epithelial development during pregnancy. Our and others' previous data have suggested that adipo-epithelial transdifferentiation could play a key role in the mammary gland alveolar development. In this study, we tested whether adipo-epithelial transdifferentiation occurs in vitro. Data show that, under appropriate co-culture conditions with mammary epithelial organoids (MEOs), mature adipocytes lose their phenotype and acquire an epithelial one. Interestingly, even in the absence of MEOs, extracellular matrix and diffusible growth factors are able to promote adipo-epithelial transdifferentiation. Gene and protein expression studies indicate that transdifferentiating adipocytes exhibit some characteristics of milk-secreting alveolar glands, including significantly higher expression of milk proteins such as whey acidic protein and β-casein. Similar data were also obtained in cultured human multipotent adipose-derived stem cell adipocytes. A miRNA sequencing experiment on the supernatant highlighted mir200c, which has a well-established role in the mesenchymal-epithelial transition, as a potential player in this phenomenon. Collectively, our data show that adipo-epithelial transdifferentiation can be reproduced in in vitro models where this phenomenon can be investigated at the molecular level.
Keywords: adipocytes; cell culture; cellular transdifferentiation; mammary gland; pregnancy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


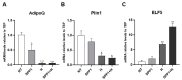
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

