Modulation of Redox and Inflammatory Signaling in Human Skin Cells Using Phytocannabinoids Applied after UVA Irradiation: In Vitro Studies
- PMID: 38891097
- PMCID: PMC11171479
- DOI: 10.3390/cells13110965
Modulation of Redox and Inflammatory Signaling in Human Skin Cells Using Phytocannabinoids Applied after UVA Irradiation: In Vitro Studies
Abstract
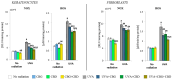
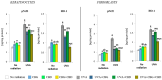
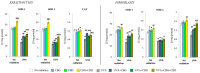
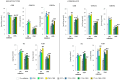
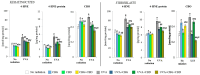
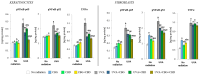
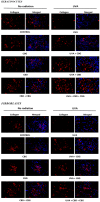
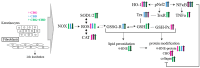
UVA exposure disturbs the metabolism of skin cells, often inducing oxidative stress and inflammation. Therefore, there is a need for bioactive compounds that limit such consequences without causing undesirable side effects. The aim of this study was to analyse in vitro the effects of the phytocannabinoids cannabigerol (CBG) and cannabidiol (CBD), which differ in terms of biological effects. Furthermore, the combined use of both compounds (CBG+CBD) has been analysed in order to increase their effectiveness in human skin fibroblasts and keratinocytes protection against UVA-induced alternation. The results obtained indicate that the effects of CBG and CBD on the redox balance might indeed be enhanced when both phytocannabinoids are applied concurrently. Those effects include a reduction in NOX activity, ROS levels, and a modification of thioredoxin-dependent antioxidant systems. The reduction in the UVA-induced lipid peroxidation and protein modification has been confirmed through lower levels of 4-HNE-protein adducts and protein carbonyl groups as well as through the recovery of collagen expression. Modification of antioxidant signalling (Nrf2/HO-1) through the administration of CBG+CBD has been proven to be associated with reduced proinflammatory signalling (NFκB/TNFα). Differential metabolic responses of keratinocytes and fibroblasts to the effects of the UVA and phytocannabinoids have indicated possible beneficial protective and regenerative effects of the phytocannabinoids, suggesting their possible application for the purpose of limiting the harmful impact of the UVA on skin cells.
Keywords: UVA radiation; fibroblasts; inflammation; keratinocytes; phytocannabinoids; redox balance.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation.Arch Dermatol Res. 2019 Apr;311(3):203-219. doi: 10.1007/s00403-019-01898-w. Epub 2019 Feb 19. Arch Dermatol Res. 2019. PMID: 30783768
-
Antioxidant and membrane-protective effects of the 3-O-ethyl ascorbic acid-cannabigerol system on UVB-irradiated human keratinocytes.Free Radic Biol Med. 2025 Feb 16;228:251-266. doi: 10.1016/j.freeradbiomed.2025.01.008. Epub 2025 Jan 6. Free Radic Biol Med. 2025. PMID: 39778604
-
The cross-talk between electrophiles, antioxidant defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation.J Dermatol Sci. 2016 Feb;81(2):107-17. doi: 10.1016/j.jdermsci.2015.11.005. Epub 2015 Dec 1. J Dermatol Sci. 2016. PMID: 26674123
-
Effect of ultraviolet radiation on the Nrf2 signaling pathway in skin cells.Int J Radiat Biol. 2021;97(10):1383-1403. doi: 10.1080/09553002.2021.1962566. Epub 2021 Aug 19. Int J Radiat Biol. 2021. PMID: 34338112 Review.
-
Comprehensive mini-review: therapeutic potential of cannabigerol - focus on the cardiovascular system.Front Pharmacol. 2025 Mar 26;16:1561385. doi: 10.3389/fphar.2025.1561385. eCollection 2025. Front Pharmacol. 2025. PMID: 40206058 Free PMC article. Review.
Cited by
-
Insights from dual-platform metabolomics on durian flowers: An alternative source of procyanidins from agricultural waste with bioactivities.Food Chem X. 2025 Jul 1;29:102719. doi: 10.1016/j.fochx.2025.102719. eCollection 2025 Jul. Food Chem X. 2025. PMID: 40686870 Free PMC article.
-
Disrupted Redox Regulation and Inflammatory Response in Pyoderma Gangrenosum.Life (Basel). 2025 Apr 6;15(4):611. doi: 10.3390/life15040611. Life (Basel). 2025. PMID: 40283167 Free PMC article.
-
Targeting Vascular and Inflammatory Crosstalk: Cannabigerol as a Dual-Pathway Modulator in Rosacea.Int J Mol Sci. 2025 Jul 16;26(14):6840. doi: 10.3390/ijms26146840. Int J Mol Sci. 2025. PMID: 40725084 Free PMC article.
References
-
- Mohd Zaid N.A., Sekar M., Bonam S.R., Gan S.H., Lum P.T., Begum M.Y., Mat Rani N.N.I., Vaijanathappa J., Wu Y.S., Subramaniyan V., et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug. Des. Devel. Ther. 2022;16:23–66. doi: 10.2147/DDDT.S326332. - DOI - PMC - PubMed
-
- Liu Y., Liu H.-Y., Li S.-H., Ma W., Wu D.-T., Li H.-B., Xiao A.-P., Liu L.-L., Zhu F., Gan R.-Y. Cannabis Sativa Bioactive Compounds and Their Extraction, Separation, Purification, and Identification Technologies: An Updated Review. TrAC Trends Anal. Chem. 2022;149:116554. doi: 10.1016/j.trac.2022.116554. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

