Interleukin-8/CXCR1 Signaling Contributes to the Progression of Pulmonary Adenocarcinoma Resulting in Malignant Pleural Effusion
- PMID: 38891100
- PMCID: PMC11172099
- DOI: 10.3390/cells13110968
Interleukin-8/CXCR1 Signaling Contributes to the Progression of Pulmonary Adenocarcinoma Resulting in Malignant Pleural Effusion
Abstract
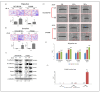
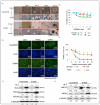
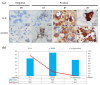
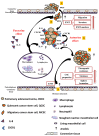
Pulmonary adenocarcinoma (PADC) treatment limited efficacy in preventing tumor progression, often resulting in malignant pleural effusion (MPE). MPE is filled with various mediators, especially interleukin-8 (IL-8). However, the role of IL-8 and its signaling mechanism within the fluid microenvironment (FME) implicated in tumor progression warrants further investigation. Primary cultured cells from samples of patients with MPE from PADC, along with a commonly utilized lung cancer cell line, were employed to examine the role of IL-8 and its receptor, CXCR1, through comparative analysis. Our study primarily assessed migration and invasion capabilities, epithelial-mesenchymal transition (EMT), and cancer stem cell (CSC) properties. Additionally, IL-8 levels in MPE fluid versus serum, along with immunohistochemical expression of IL-8/CXCR1 signaling in tumor tissue and cell blocks were analyzed. IL-8/CXCR1 overexpression enhanced EMT and CSC properties. Furthermore, the immunocytochemical examination of 17 cell blocks from patients with PADC and MPE corroborated the significant correlation between upregulated IL-8 and CXCR1 expression and the co-expression of IL-8 and CXCR1 in MPE with distant metastasis. In summary, the IL-8/ CXCR1 axis in FME is pivotal to tumor promotion via paracrine and autocrine signaling. Our study provides a therapeutic avenue for improving the prognosis of PADC patients with MPE.
Keywords: CXCR1; cancer stem cell; epithelial-mesenchymal transition; fluid microenvironment; interleukin-8; interleukin-8/CXCR1 signaling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

