Hypoxia Increases the Efficiencies of Cellular Reprogramming and Oncogenic Transformation in Human Blood Cell Subpopulations In Vitro and In Vivo
- PMID: 38891103
- PMCID: PMC11172288
- DOI: 10.3390/cells13110971
Hypoxia Increases the Efficiencies of Cellular Reprogramming and Oncogenic Transformation in Human Blood Cell Subpopulations In Vitro and In Vivo
Abstract
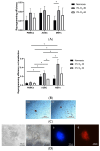
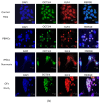
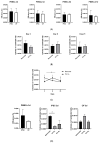
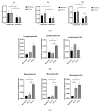
Patients with chronic hypoxia show a higher tumor incidence; however, no primary common cause has been recognized. Given the similarities between cellular reprogramming and oncogenic transformation, we directly compared these processes in human cells subjected to hypoxia. Mouse embryonic fibroblasts were employed as controls to compare transfection and reprogramming efficiency; human adipose-derived mesenchymal stem cells were employed as controls in human cells. Easily obtainable human peripheral blood mononuclear cells (PBMCs) were chosen to establish a standard protocol to compare cell reprogramming (into induced pluripotent stem cells (iPSCs)) and oncogenic focus formation efficiency. Cell reprogramming was achieved for all three cell types, generating actual pluripotent cells capable for differentiating into the three germ layers. The efficiencies of the cell reprogramming and oncogenic transformation were similar. Hypoxia slightly increased the reprogramming efficiency in all the cell types but with no statistical significance for PBMCs. Various PBMC types can respond to hypoxia differently; lymphocytes and monocytes were, therefore, reprogrammed separately, finding a significant difference between normoxia and hypoxia in monocytes in vitro. These differences were then searched for in vivo. The iPSCs and oncogenic foci were generated from healthy volunteers and patients with chronic obstructive pulmonary disease (COPD). Although higher iPSC generation efficiency in the patients with COPD was found for lymphocytes, this increase was not statistically significant for oncogenic foci. Remarkably, a higher statistically significant efficiency in COPD monocytes was demonstrated for both processes, suggesting that physiological hypoxia exerts an effect on cell reprogramming and oncogenic transformation in vivo in at least some cell types.
Keywords: adipose-derived stem cells; cellular reprograming; chronic obstructive pulmonary disease; hypoxia; induced pluripotent stem cells; peripheral blood mononuclear cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Efficient Reprogramming of Canine Peripheral Blood Mononuclear Cells into Induced Pluripotent Stem Cells.Stem Cells Dev. 2021 Jan 15;30(2):79-90. doi: 10.1089/scd.2020.0084. Epub 2020 Dec 24. Stem Cells Dev. 2021. PMID: 33256572
-
Induced pluripotency and oncogenic transformation are related processes.Stem Cells Dev. 2013 Jan 1;22(1):37-50. doi: 10.1089/scd.2012.0375. Epub 2012 Oct 26. Stem Cells Dev. 2013. PMID: 22998387 Free PMC article.
-
Efficient generation of induced pluripotent stem cell lines from peripheral blood mononuclear cells.Stem Cell Res. 2023 Jun;69:103088. doi: 10.1016/j.scr.2023.103088. Epub 2023 Mar 28. Stem Cell Res. 2023. PMID: 37099933
-
An Overview on Promising Somatic Cell Sources Utilized for the Efficient Generation of Induced Pluripotent Stem Cells.Stem Cell Rev Rep. 2021 Dec;17(6):1954-1974. doi: 10.1007/s12015-021-10200-3. Epub 2021 Jun 7. Stem Cell Rev Rep. 2021. PMID: 34100193 Review.
-
Tissue-Restricted Stem Cells as Starting Cell Source for Efficient Generation of Pluripotent Stem Cells: An Overview.Adv Exp Med Biol. 2022;1376:151-180. doi: 10.1007/5584_2021_660. Adv Exp Med Biol. 2022. PMID: 34611861 Review.
Cited by
-
Acute Severe Hypoxia Decreases Mitochondrial Chain Complex II Respiration in Human Peripheral Blood Mononuclear Cells.Int J Mol Sci. 2025 Jan 15;26(2):705. doi: 10.3390/ijms26020705. Int J Mol Sci. 2025. PMID: 39859418 Free PMC article.
References
-
- Figueira Gonçalves J.M., Dorta Sánchez R., Pérez Méndez L.I., Pérez Negrín L., García-Talavera I., Pérez Rodríguez A., Díaz Pérez D., Viña Manrique P., Guzmán Sáenz C. Incidence of Cancer in Outpatients with Chronic Obstructive Pulmonary Disease. Rev. Clin. Esp. Engl. Ed. 2017;217:387–393. doi: 10.1016/j.rce.2017.06.004. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

