A Novel CRISPR-Cas9 Strategy to Target DYSTROPHIN Mutations Downstream of Exon 44 in Patient-Specific DMD iPSCs
- PMID: 38891104
- PMCID: PMC11171783
- DOI: 10.3390/cells13110972
A Novel CRISPR-Cas9 Strategy to Target DYSTROPHIN Mutations Downstream of Exon 44 in Patient-Specific DMD iPSCs
Abstract
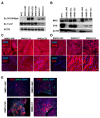
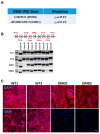
Mutations in the DMD gene cause fatal Duchenne Muscular Dystrophy (DMD). An attractive therapeutic approach is autologous cell transplantation utilizing myogenic progenitors derived from induced pluripotent stem cells (iPSCs). Given that a significant number of DMD mutations occur between exons 45 and 55, we developed a gene knock-in approach to correct any mutations downstream of exon 44. We applied this approach to two DMD patient-specific iPSC lines carrying mutations in exons 45 and 51 and confirmed mini-DYSTROPHIN (mini-DYS) protein expression in corrected myotubes by western blot and immunofluorescence staining. Transplantation of gene-edited DMD iPSC-derived myogenic progenitors into NSG/mdx4Cv mice produced donor-derived myofibers, as shown by the dual expression of human DYSTROPHIN and LAMIN A/C. These findings further provide proof-of-concept for the use of programmable nucleases for the development of autologous iPSC-based therapy for muscular dystrophies.
Keywords: CRISPR-Cas9; DMD; dystrophin; gene editing; patient-specific iPS cells; transplantation.
Conflict of interest statement
R.C.R.P. is cofounder and holds equity in Myogenica. All other authors have no competing financial interests.
Figures



References
-
- Mias-Lucquin D., Dos Santos Morais R., Chéron A., Lagarrigue M., Winder S.J., Chenuel T., Pérez J., Appavou M.-S., Martel A., Alviset G., et al. How the central domain of dystrophin acts to bridge F-actin to sarcolemmal lipids. J. Struct. Biol. 2020;209:107411. doi: 10.1016/j.jsb.2019.107411. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

