Specific Cell Targeting by Toxoplasma gondii Displaying Functional Single-Chain Variable Fragment as a Novel Strategy; A Proof of Principle
- PMID: 38891106
- PMCID: PMC11172386
- DOI: 10.3390/cells13110975
Specific Cell Targeting by Toxoplasma gondii Displaying Functional Single-Chain Variable Fragment as a Novel Strategy; A Proof of Principle
Abstract
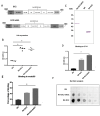
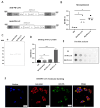
Toxoplasma gondii holds significant therapeutic potential; however, its nonspecific invasiveness results in off-target effects. The purpose of this study is to evaluate whether T. gondii specificity can be improved by surface display of scFv directed against dendritic cells' endocytic receptor, DEC205, and immune checkpoint PD-L1. Anti-DEC205 scFv was anchored to the T. gondii surface either directly via glycosylphosphatidylinositol (GPI) or by fusion with the SAG1 protein. Both constructs were successfully expressed, but the binding results suggested that the anti-DEC-SAG1 scFv had more reliable functionality towards recombinant DEC protein and DEC205-expressing MutuDC cells. Two anti-PD-L1 scFv constructs were developed that differed in the localization of the HA tag. Both constructs were adequately expressed, but the localization of the HA tag determined the functionality by binding to PD-L1 protein. Co-incubation of T. gondii displaying anti-PD-L1 scFv with tumor cells expressing/displaying different levels of PD-L1 showed strong binding depending on the level of available biomarker. Neutralization assays confirmed that binding was due to the specific interaction between anti-PD-L1 scFv and its ligand. A mixed-cell assay showed that T. gondii expressing anti-PD-L1 scFv predominately targets the PD-L1-positive cells, with negligible off-target binding. The recombinant RH-PD-L1-C strain showed increased killing ability on PD-L1+ tumor cell lines compared to the parental strain. Moreover, a co-culture assay of target tumor cells and effector CD8+ T cells showed that our model could inhibit PD1/PD-L1 interaction and potentiate T-cell immune response. These findings highlight surface display of antibody fragments as a promising strategy of targeting replicative T. gondii strains while minimizing nonspecific binding.
Keywords: PD-L1; Toxoplasma gondii; cancer immunotherapy; immune checkpoint; scFv; surface display; targeting.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

