UV-B Radiation Disrupts Membrane Lipid Organization and Suppresses Protein Mobility of GmNARK in Arabidopsis
- PMID: 38891343
- PMCID: PMC11174901
- DOI: 10.3390/plants13111536
UV-B Radiation Disrupts Membrane Lipid Organization and Suppresses Protein Mobility of GmNARK in Arabidopsis
Abstract
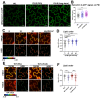
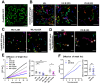
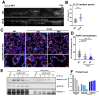
While it is well known that plants interpret UV-B as an environmental cue and a potential stressor influencing their growth and development, the specific effects of UV-B-induced oxidative stress on the dynamics of membrane lipids and proteins remain underexplored. Here, we demonstrate that UV-B exposure notably increases the formation of ordered lipid domains on the plasma membrane (PM) and significantly alters the behavior of the Glycine max nodule autoregulation receptor kinase (GmNARK) protein in Arabidopsis leaves. The GmNARK protein was located on the PM and accumulated as small particles in the cytoplasm. We found that UV-B irradiation interrupted the lateral diffusion of GmNARK proteins on the PM. Furthermore, UV-B light decreases the efficiency of surface molecule internalization by clathrin-mediated endocytosis (CME). In brief, UV-B irradiation increased the proportion of the ordered lipid phase and disrupted clathrin-dependent endocytosis; thus, the endocytic trafficking and lateral mobility of GmNARK protein on the plasma membrane are crucial for nodule formation tuning. Our results revealed a novel role of low-intensity UV-B stress in altering the organization of the plasma membrane and the dynamics of membrane-associated proteins.
Keywords: GmNARK; UV-B; endocytosis; lipid organization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The Soybean GmNARK Affects ABA and Salt Responses in Transgenic Arabidopsis thaliana.Front Plant Sci. 2018 Apr 18;9:514. doi: 10.3389/fpls.2018.00514. eCollection 2018. Front Plant Sci. 2018. PMID: 29720993 Free PMC article.
-
Investigation of downstream signals of the soybean autoregulation of nodulation receptor kinase GmNARK.Mol Plant Microbe Interact. 2008 Oct;21(10):1337-48. doi: 10.1094/MPMI-21-10-1337. Mol Plant Microbe Interact. 2008. PMID: 18785829
-
Cross-talk between clathrin-dependent post-Golgi trafficking and clathrin-mediated endocytosis in Arabidopsis root cells.Plant Cell. 2021 Sep 24;33(9):3057-3075. doi: 10.1093/plcell/koab180. Plant Cell. 2021. PMID: 34240193 Free PMC article.
-
Change your TPLATE, change your fate: plant CME and beyond.Trends Plant Sci. 2015 Jan;20(1):41-8. doi: 10.1016/j.tplants.2014.09.002. Epub 2014 Sep 30. Trends Plant Sci. 2015. PMID: 25278268 Review.
-
Membrane remodeling in clathrin-mediated endocytosis.J Cell Sci. 2018 Sep 3;131(17):jcs216812. doi: 10.1242/jcs.216812. J Cell Sci. 2018. PMID: 30177505 Review.
References
LinkOut - more resources
Full Text Sources

