Dynamic Nonlinear Spatial Integrations on Encoding Contrasting Stimuli of Tectal Neurons
- PMID: 38891623
- PMCID: PMC11171053
- DOI: 10.3390/ani14111577
Dynamic Nonlinear Spatial Integrations on Encoding Contrasting Stimuli of Tectal Neurons
Abstract
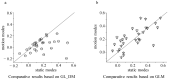
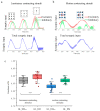
Animals detect targets using a variety of visual cues, with the visual salience of these cues determining which environmental features receive priority attention and further processing. Surround modulation plays a crucial role in generating visual saliency, which has been extensively studied in avian tectal neurons. Recent work has reported that the suppression of tectal neurons induced by motion contrasting stimulus is stronger than that by luminance contrasting stimulus. However, the underlying mechanism remains poorly understood. In this study, we built a computational model (called Generalized Linear-Dynamic Modulation) which incorporates independent nonlinear tuning mechanisms for excitatory and inhibitory inputs. This model aims to describe how tectal neurons encode contrasting stimuli. The results showed that: (1) The dynamic nonlinear integration structure substantially improved the accuracy (significant difference (p < 0.001, paired t-test) in the goodness of fit between the two models) of the predicted responses to contrasting stimuli, verifying the nonlinear processing performed by tectal neurons. (2) The modulation difference between luminance and motion contrasting stimuli emerged from the predicted response by the full model but not by that with only excitatory synaptic input (spatial luminance: 89 ± 2.8% (GL_DM) vs. 87 ± 2.1% (GL_DMexc); motion contrasting stimuli: 87 ± 1.7% (GL_DM) vs. 83 ± 2.2% (GL_DMexc)). These results validate the proposed model and further suggest the role of dynamic nonlinear spatial integrations in contextual visual information processing, especially in spatial integration, which is important for object detection performed by birds.
Keywords: contrasting stimuli; dynamic nonlinear spatial integrations; optic tectum; surround modulation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Comparison of pop-out responses to luminance and motion contrasting stimuli of tectal neurons in pigeons.Brain Res. 2020 Nov 15;1747:147068. doi: 10.1016/j.brainres.2020.147068. Epub 2020 Aug 19. Brain Res. 2020. PMID: 32827547
-
Responses of tectal neurons to contrasting stimuli: an electrophysiological study in the barn owl.PLoS One. 2012;7(6):e39559. doi: 10.1371/journal.pone.0039559. Epub 2012 Jun 20. PLoS One. 2012. PMID: 22745787 Free PMC article.
-
Inhibition to excitation ratio regulates visual system responses and behavior in vivo.J Neurophysiol. 2011 Nov;106(5):2285-302. doi: 10.1152/jn.00641.2011. Epub 2011 Jul 27. J Neurophysiol. 2011. PMID: 21795628 Free PMC article.
-
The nucleus isthmi and dual modulation of the receptive field of tectal neurons in non-mammals.Brain Res Brain Res Rev. 2003 Jan;41(1):13-25. doi: 10.1016/s0165-0173(02)00217-5. Brain Res Brain Res Rev. 2003. PMID: 12505645 Review.
-
Functional anatomy of the tectum mesencephali of the goldfish. An explorative analysis of the functional implications of the laminar structural organization of the tectum.Brain Res. 1983 Dec;287(3):247-97. doi: 10.1016/0165-0173(83)90008-5. Brain Res. 1983. PMID: 6362772 Review.
Cited by
-
Cognivue Clarity® characterizes amyloid status and preclinical Alzheimer's disease in biomarker confirmed cohorts in the Bio-Hermes Study.J Alzheimers Dis. 2025 Mar;104(1):83-94. doi: 10.1177/13872877251314117. Epub 2025 Jan 26. J Alzheimers Dis. 2025. PMID: 39865688 Free PMC article.
References
-
- Tadin D. Strong evidence against a common center-surround mechanism in visual processing. J. Vis. 2022;22:58. doi: 10.1167/jov.22.3.58. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

