Unveiling the Nature and Strength of Selenium-Centered Chalcogen Bonds in Binary Complexes of SeO2 with Oxygen-/Sulfur-Containing Lewis Bases: Insights from Theoretical Calculations
- PMID: 38891796
- PMCID: PMC11171880
- DOI: 10.3390/ijms25115609
Unveiling the Nature and Strength of Selenium-Centered Chalcogen Bonds in Binary Complexes of SeO2 with Oxygen-/Sulfur-Containing Lewis Bases: Insights from Theoretical Calculations
Abstract
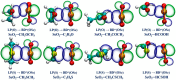
Among various non-covalent interactions, selenium-centered chalcogen bonds (SeChBs) have garnered considerable attention in recent years as a result of their important contributions to crystal engineering, organocatalysis, molecular recognition, materials science, and biological systems. Herein, we systematically investigated π-hole-type Se∙∙∙O/S ChBs in the binary complexes of SeO2 with a series of O-/S-containing Lewis bases by means of high-level ab initio computations. The results demonstrate that there exists an attractive interaction between the Se atom of SeO2 and the O/S atom of Lewis bases. The interaction energies computed at the MP2/aug-cc-pVTZ level range from -4.68 kcal/mol to -10.83 kcal/mol for the Se∙∙∙O chalcogen-bonded complexes and vary between -3.53 kcal/mol and -13.77 kcal/mol for the Se∙∙∙S chalcogen-bonded complexes. The Se∙∙∙O/S ChBs exhibit a relatively short binding distance in comparison to the sum of the van der Waals radii of two chalcogen atoms. The Se∙∙∙O/S ChBs in all of the studied complexes show significant strength and a closed-shell nature, with a partially covalent character in most cases. Furthermore, the strength of these Se∙∙∙O/S ChBs generally surpasses that of the C/O-H∙∙∙O hydrogen bonds within the same complex. It should be noted that additional C/O-H∙∙∙O interactions have a large effect on the geometric structures and strength of Se∙∙∙O/S ChBs. Two subunits are connected together mainly via the orbital interaction between the lone pair of O/S atoms in the Lewis bases and the BD*(OSe) anti-bonding orbital of SeO2, except for the SeO2∙∙∙HCSOH complex. The electrostatic component emerges as the largest attractive contributor for stabilizing the examined complexes, with significant contributions from induction and dispersion components as well.
Keywords: binary clusters; chalcogen bonds; quantum chemical calculations; π–hole interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
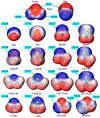
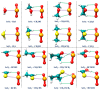
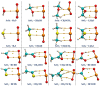
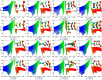
References
MeSH terms
Substances
Grants and funding
- 22263003/the National Natural Science Foundation of China
- ZK2024-145, ZK2022-369, ZK2022-406/the Natural Science Foundation of Guizhou Province
- 2022-188/the Scientific Research Project (Youth Project) of Guizhou Colleges and Universities
- KY2022-222/the Youth Science and Technology Talents Growth Project of Guizhou Ordinary Colleges and Universities
- gzwkj2022-513/the Science and Technology Fund Project of Guizhou Provincial Health Commission
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

