Combination of FOLFOXIRI Drugs with Oncolytic Coxsackie B3 Virus PD-H Synergistically Induces Oncolysis in the Refractory Colorectal Cancer Cell Line Colo320
- PMID: 38891807
- PMCID: PMC11171967
- DOI: 10.3390/ijms25115618
Combination of FOLFOXIRI Drugs with Oncolytic Coxsackie B3 Virus PD-H Synergistically Induces Oncolysis in the Refractory Colorectal Cancer Cell Line Colo320
Abstract
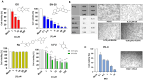
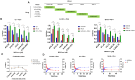
FOLFOXIRI chemotherapy is a first-line therapy for advanced or metastatic colorectal cancer (CRC), yet its therapeutic efficacy remains limited. Immunostimulatory therapies like oncolytic viruses can complement chemotherapies by fostering the infiltration of the tumor by immune cells and enhancing drug cytotoxicity. In this study, we explored the effect of combining the FOLFOXIRI chemotherapeutic agents with the oncolytic coxsackievirus B3 (CVB3) PD-H in the CRC cell line Colo320. Additionally, we examined the impact of the drugs on the expression of microRNAs (miRs), which could be used to increase the safety of oncolytic CVB3 containing corresponding miR target sites (miR-TS). The measurement of cytotoxic activity using the Chou-Talalay combination index approach revealed that PD-H synergistically enhanced the cytotoxic activity of oxaliplatin (OX), 5-fluorouracil (5-FU) and SN-38. PD-H replication was not affected by OX and SN-38 but inhibited by high concentrations of 5-FU. MiR expression levels were not or only slightly elevated by the drugs or with drug/PD-H combinations on Colo320 cells. Moreover, the drug treatment did not increase the mutation rate of the miR-TS inserted into the PD-H genome. The results demonstrate that the combination of FOLFOXIRI drugs and PD-H may be a promising approach to enhance the therapeutic effect of FOLFOXIRI therapy in CRC.
Keywords: chemotherapy; colorectal cancer; combination therapy; coxsackievirus; oncolytic virus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
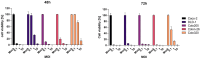


References
-
- Hossain M.S., Karuniawati H., Jairoun A.A., Urbi Z., Ooi D.J., John A., Lim Y.C., Kibria K.M.K., Mohiuddin A.K.M., Ming L.C., et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers. 2022;14:1732. doi: 10.3390/cancers14071732. - DOI - PMC - PubMed
-
- Krepelkova I. Colorectal Cancer—More People Screened, More Lives Saved. [(accessed on 29 July 2019)]. Available online: https://www.biovendor.com/colorectal-cancer--more-people-screened-more-l....
-
- Edwards B.K., Ward E., Kohler B.A., Eheman C., Zauber A.G., Anderson R.N., Jemal A., Schymura M.J., Lansdorp-Vogelaar I., Seeff L.C., et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. - DOI - PMC - PubMed
-
- Falcone A., Masi G., Allegrini G., Danesi R., Pfanner E., Brunetti I.M., Di Paolo A., Cupini S., Del Tacca M., Conte P. Biweekly chemotherapy with oxaliplatin, irinotecan, infusional Fluorouracil, and leucovorin: A pilot study in patients with metastatic colorectal cancer. J. Clin. Oncol. 2002;20:4006–4014. doi: 10.1200/JCO.2002.12.075. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

