Metabolic Dysfunction-Associated Steatotic Liver Disease: From Pathogenesis to Current Therapeutic Options
- PMID: 38891828
- PMCID: PMC11172019
- DOI: 10.3390/ijms25115640
Metabolic Dysfunction-Associated Steatotic Liver Disease: From Pathogenesis to Current Therapeutic Options
Abstract
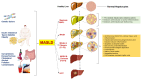
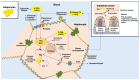
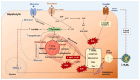
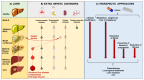
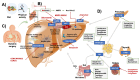
The epidemiological burden of liver steatosis associated with metabolic diseases is continuously growing worldwide and in all age classes. This condition generates possible progression of liver damage (i.e., inflammation, fibrosis, cirrhosis, hepatocellular carcinoma) but also independently increases the risk of cardio-metabolic diseases and cancer. In recent years, the terminological evolution from "nonalcoholic fatty liver disease" (NAFLD) to "metabolic dysfunction-associated fatty liver disease" (MAFLD) and, finally, "metabolic dysfunction-associated steatotic liver disease" (MASLD) has been paralleled by increased knowledge of mechanisms linking local (i.e., hepatic) and systemic pathogenic pathways. As a consequence, the need for an appropriate classification of individual phenotypes has been oriented to the investigation of innovative therapeutic tools. Besides the well-known role for lifestyle change, a number of pharmacological approaches have been explored, ranging from antidiabetic drugs to agonists acting on the gut-liver axis and at a systemic level (mainly farnesoid X receptor (FXR) agonists, PPAR agonists, thyroid hormone receptor agonists), anti-fibrotic and anti-inflammatory agents. The intrinsically complex pathophysiological history of MASLD makes the selection of a single effective treatment a major challenge, so far. In this evolving scenario, the cooperation between different stakeholders (including subjects at risk, health professionals, and pharmaceutical industries) could significantly improve the management of disease and the implementation of primary and secondary prevention measures. The high healthcare burden associated with MASLD makes the search for new, effective, and safe drugs a major pressing need, together with an accurate characterization of individual phenotypes. Recent and promising advances indicate that we may soon enter the era of precise and personalized therapy for MASLD/MASH.
Keywords: MAFLD; MASLD; NAFLD; NASH; clinical trials; drug therapy; fatty liver; liver fibrosis.
Conflict of interest statement
Author Valeria Idone was employed by the company Aboca S.p.a. Author Annarita Graziani was employed by the company AllergoSan Pharmazeutische Produkte Forschungs- und Vertriebs GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Finucane M.M., Stevens G.A., Cowan M.J., Danaei G., Lin J.K., Paciorek C.J., Singh G.M., Gutierrez H.R., Lu Y.A., Bahalim A.N., et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. - DOI - PMC - PubMed
-
- World Health Organization Obesity and Overweight. [(accessed on 23 February 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

