Structural and Interactional Analysis of the Flavonoid Pathway Proteins: Chalcone Synthase, Chalcone Isomerase and Chalcone Isomerase-like Protein
- PMID: 38891840
- PMCID: PMC11172311
- DOI: 10.3390/ijms25115651
Structural and Interactional Analysis of the Flavonoid Pathway Proteins: Chalcone Synthase, Chalcone Isomerase and Chalcone Isomerase-like Protein
Abstract
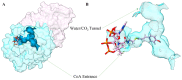
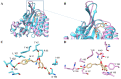
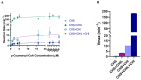
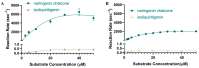
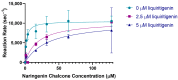
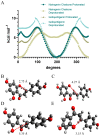
Chalcone synthase (CHS) and chalcone isomerase (CHI) catalyze the first two committed steps of the flavonoid pathway that plays a pivotal role in the growth and reproduction of land plants, including UV protection, pigmentation, symbiotic nitrogen fixation, and pathogen resistance. Based on the obtained X-ray crystal structures of CHS, CHI, and chalcone isomerase-like protein (CHIL) from the same monocotyledon, Panicum virgatum, along with the results of the steady-state kinetics, spectroscopic/thermodynamic analyses, intermolecular interactions, and their effect on each catalytic step are proposed. In addition, PvCHI's unique activity for both naringenin chalcone and isoliquiritigenin was analyzed, and the observed hierarchical activity for those type-I and -II substrates was explained with the intrinsic characteristics of the enzyme and two substrates. The structure of PvCHS complexed with naringenin supports uncompetitive inhibition. PvCHS displays intrinsic catalytic promiscuity, evident from the formation of p-coumaroyltriacetic acid lactone (CTAL) in addition to naringenin chalcone. In the presence of PvCHIL, conversion of p-coumaroyl-CoA to naringenin through PvCHS and PvCHI displayed ~400-fold increased Vmax with reduced formation of CTAL by 70%. Supporting this model, molecular docking, ITC (Isothermal Titration Calorimetry), and FRET (Fluorescence Resonance Energy Transfer) indicated that both PvCHI and PvCHIL interact with PvCHS in a non-competitive manner, indicating the plausible allosteric effect of naringenin on CHS. Significantly, the presence of naringenin increased the affinity between PvCHS and PvCHIL, whereas naringenin chalcone decreased the affinity, indicating a plausible feedback mechanism to minimize spontaneous incorrect stereoisomers. These are the first findings from a three-body system from the same species, indicating the importance of the macromolecular assembly of CHS-CHI-CHIL in determining the amount and type of flavonoids produced in plant cells.
Keywords: Panicum virgatum; Sorghum bicolor; anthocyanidins; anthocyanin; flavones; flavonols; metabolon.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Regulatory ligand binding in plant chalcone isomerase-like (CHIL) proteins.J Biol Chem. 2023 Jun;299(6):104804. doi: 10.1016/j.jbc.2023.104804. Epub 2023 May 10. J Biol Chem. 2023. PMID: 37172720 Free PMC article.
-
A conserved strategy of chalcone isomerase-like protein to rectify promiscuous chalcone synthase specificity.Nat Commun. 2020 Feb 13;11(1):870. doi: 10.1038/s41467-020-14558-9. Nat Commun. 2020. PMID: 32054839 Free PMC article.
-
Structural insights into catalytic promiscuity of chalcone synthase from Glycine max (L.) Merr.: Coenzyme A-induced alteration of product specificity.Biochem Biophys Res Commun. 2024 Jul 23;718:150080. doi: 10.1016/j.bbrc.2024.150080. Epub 2024 May 8. Biochem Biophys Res Commun. 2024. PMID: 38735137
-
Elusive partners: a review of the auxiliary proteins guiding metabolic flux in flavonoid biosynthesis.Plant J. 2021 Oct;108(2):314-329. doi: 10.1111/tpj.15446. Epub 2021 Aug 11. Plant J. 2021. PMID: 34318549 Review.
-
Increasing antioxidant levels in tomatoes through modification of the flavonoid biosynthetic pathway.J Exp Bot. 2002 Oct;53(377):2099-106. doi: 10.1093/jxb/erf044. J Exp Bot. 2002. PMID: 12324533 Review.
Cited by
-
Bioreduction of 4'-Hydroxychalcone in Deep Eutectic Solvents: Optimization and Efficacy with Various Yeast Strains.Int J Mol Sci. 2024 Jun 28;25(13):7152. doi: 10.3390/ijms25137152. Int J Mol Sci. 2024. PMID: 39000255 Free PMC article.
-
Functional analysis of type II chalcone isomerase (CHI) genes in regulating soybean (Glycine max L.) nodule formation.GM Crops Food. 2025 Dec;16(1):305-317. doi: 10.1080/21645698.2025.2486280. Epub 2025 Mar 31. GM Crops Food. 2025. PMID: 40165359 Free PMC article.
-
Biochemical evaluation of molecular parts for flavonoid production using plant synthetic biology.Front Plant Sci. 2025 Apr 15;16:1528122. doi: 10.3389/fpls.2025.1528122. eCollection 2025. Front Plant Sci. 2025. PMID: 40303856 Free PMC article. Review.
-
Approach for elucidating the structure of phenolics in tomato.Food Chem X. 2025 Aug 8;30:102907. doi: 10.1016/j.fochx.2025.102907. eCollection 2025 Aug. Food Chem X. 2025. PMID: 40831965 Free PMC article.
-
Evolutionary landscape of plant chalcone isomerase-fold gene families.Front Plant Sci. 2025 Mar 28;16:1559547. doi: 10.3389/fpls.2025.1559547. eCollection 2025. Front Plant Sci. 2025. PMID: 40225028 Free PMC article.
References
-
- Guetsky R., Kobiler I., Wang X., Perlman N., Gollop N., Avila-Quezada G., Hadar I., Prusky D. Metabolism of the Flavonoid Epicatechin by Laccase of Colletotrichum gloeosporioides and Its Effect on Pathogenicity on Avocado Fruits. Phytopathology. 2005;95:1341–1348. doi: 10.1094/phyto-95-1341. - DOI - PubMed
-
- Francis G.J.L. Jack Natural Food Colorants: Science and Technology. CRC Press; Boca Raton, FL, USA: 2014.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

