How Much Do You Fuse? A Comparison of Cell Fusion Assays in a Breast Cancer Model
- PMID: 38891857
- PMCID: PMC11172233
- DOI: 10.3390/ijms25115668
How Much Do You Fuse? A Comparison of Cell Fusion Assays in a Breast Cancer Model
Abstract
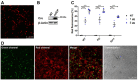
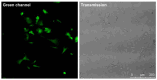
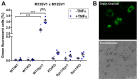
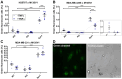
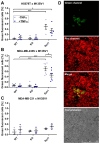
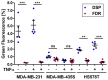
Cell fusion is a biological process that is crucial for the development and homeostasis of different tissues, but it is also pathophysiologically associated with tumor progression and malignancy. The investigation of cell fusion processes is difficult because there is no standardized marker. Many studies therefore use different systems to observe and quantify cell fusion in vitro and in vivo. The comparability of the results must be critically questioned, because both the experimental procedure and the assays differ between studies. The comparability of the fluorescence-based fluorescence double reporter (FDR) and dual split protein (DSP) assay was investigated as part of this study, in which general conditions were kept largely constant. In order to be able to induce both a high and a low cell fusion rate, M13SV1 breast epithelial cells were modified with regard to the expression level of the fusogenic protein Syncytin-1 and its receptor ASCT2 and were co-cultivated for 72 h with different breast cancer cell lines. A high number of fused cells was found in co-cultures with Syncytin-1-overexpressing M13SV1 cells, but differences between the assays were also observed. This shows that the quantification of cell fusion events in particular is highly dependent on the assay selected, but the influence of fusogenic proteins can be visualized very well.
Keywords: ASCT2; Syncytin-1; breast cancer; cell fusion; dual split protein assay; fluorescence double reporter assay; fluorescence-based cell fusion assays; quantification of cell fusion.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Matrix metalloproteinase-9 (MMP9) is involved in the TNF-α-induced fusion of human M13SV1-Cre breast epithelial cells and human MDA-MB-435-pFDR1 cancer cells.Cell Commun Signal. 2018 Apr 10;16(1):14. doi: 10.1186/s12964-018-0226-1. Cell Commun Signal. 2018. PMID: 29636110 Free PMC article.
-
Heterologous avian system for quantitative analysis of Syncytin-1 interaction with ASCT2 receptor.Retrovirology. 2021 Jun 22;18(1):15. doi: 10.1186/s12977-021-00558-0. Retrovirology. 2021. PMID: 34158079 Free PMC article.
-
Identification of an endogenous retroviral envelope gene with fusogenic activity and placenta-specific expression in the rabbit: a new "syncytin" in a third order of mammals.Retrovirology. 2009 Nov 27;6:107. doi: 10.1186/1742-4690-6-107. Retrovirology. 2009. PMID: 19943933 Free PMC article.
-
Syncytin: the major regulator of trophoblast fusion? Recent developments and hypotheses on its action.Hum Reprod Update. 2004 Nov-Dec;10(6):487-96. doi: 10.1093/humupd/dmh039. Epub 2004 Aug 27. Hum Reprod Update. 2004. PMID: 15333590 Review.
-
Epigenetic and non-epigenetic regulation of syncytin-1 expression in human placenta and cancer tissues.Cell Signal. 2014 Mar;26(3):648-56. doi: 10.1016/j.cellsig.2013.11.002. Epub 2013 Nov 9. Cell Signal. 2014. PMID: 24216608 Review.
Cited by
-
Effects of polystyrene nano- and microplastics on human breast epithelial cells and human breast cancer cells.Heliyon. 2024 Oct 4;10(20):e38686. doi: 10.1016/j.heliyon.2024.e38686. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39449700 Free PMC article.
References
-
- Dittmar T., Zänker K.S. In: Cell Fusion in Health and Disease; Volume I: Cell Fusion in Health. Dittmar T., Zänker K.S., editors. Springer; Dordrecht, The Netherlands: 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

