Neuromodulatory Responses Elicited by Intermittent versus Continuous Transcranial Focused Ultrasound Stimulation of the Motor Cortex in Rats
- PMID: 38891875
- PMCID: PMC11171676
- DOI: 10.3390/ijms25115687
Neuromodulatory Responses Elicited by Intermittent versus Continuous Transcranial Focused Ultrasound Stimulation of the Motor Cortex in Rats
Abstract
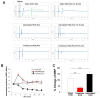
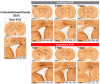
Transcranial focused ultrasound stimulation (tFUS) has emerged as a promising neuromodulation technique that delivers acoustic energy with high spatial resolution for inducing long-term potentiation (LTP)- or depression (LTD)-like plasticity. The variability in the primary effects of tFUS-induced plasticity could be due to different stimulation patterns, such as intermittent versus continuous, and is an aspect that requires further detailed exploration. In this study, we developed a platform to evaluate the neuromodulatory effects of intermittent and continuous tFUS on motor cortical plasticity before and after tFUS application. Three groups of rats were exposed to either intermittent, continuous, or sham tFUS. We analyzed the neuromodulatory effects on motor cortical excitability by examining changes in motor-evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS). We also investigated the effects of different stimulation patterns on excitatory and inhibitory neural biomarkers, examining c-Fos and glutamic acid decarboxylase (GAD-65) expression using immunohistochemistry staining. Additionally, we evaluated the safety of tFUS by analyzing glial fibrillary acidic protein (GFAP) expression. The current results indicated that intermittent tFUS produced a facilitation effect on motor excitability, while continuous tFUS significantly inhibited motor excitability. Furthermore, neither tFUS approach caused injury to the stimulation sites in rats. Immunohistochemistry staining revealed increased c-Fos and decreased GAD-65 expression following intermittent tFUS. Conversely, continuous tFUS downregulated c-Fos and upregulated GAD-65 expression. In conclusion, our findings demonstrate that both intermittent and continuous tFUS effectively modulate cortical excitability. The neuromodulatory effects may result from the activation or deactivation of cortical neurons following tFUS intervention. These effects are considered safe and well-tolerated, highlighting the potential for using different patterns of tFUS in future clinical neuromodulatory applications.
Keywords: motor-evoked potentials; neuromodulation; plasticity; rats; transcranial focused ultrasound.
Conflict of interest statement
H.-L.L. served as a technical consultant at NaviFUS Corp., Taipei, Taiwan and currently holds several therapeutic ultrasound-related patents; P.-C.C. concurrently served as a part-time research and development scientist at NaviFUS Corp., Taiwan.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

