Recent Progress in Innate Immune Responses to Enterovirus A71 and Viral Evasion Strategies
- PMID: 38891876
- PMCID: PMC11172324
- DOI: 10.3390/ijms25115688
Recent Progress in Innate Immune Responses to Enterovirus A71 and Viral Evasion Strategies
Abstract
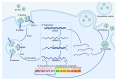
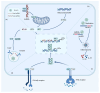
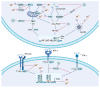
Enterovirus A71 (EV-A71) is a major pathogen causing hand, foot, and mouth disease (HFMD) in children worldwide. It can lead to severe gastrointestinal, pulmonary, and neurological complications. The innate immune system, which rapidly detects pathogens via pathogen-associated molecular patterns or pathogen-encoded effectors, serves as the first defensive line against EV-A71 infection. Concurrently, the virus has developed various sophisticated strategies to evade host antiviral responses and establish productive infection. Thus, the virus-host interactions and conflicts, as well as the ability to govern biological events at this first line of defense, contribute significantly to the pathogenesis and outcomes of EV-A71 infection. In this review, we update recent progress on host innate immune responses to EV-A71 infection. In addition, we discuss the underlying strategies employed by EV-A71 to escape host innate immune responses. A better understanding of the interplay between EV-A71 and host innate immunity may unravel potential antiviral targets, as well as strategies that can improve patient outcomes.
Keywords: enterovirus; evasion strategies; hand, foot, and mouth disease (HFMD); innate immune response.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Immune responses against enterovirus A71 infection: Implications for vaccine success.Rev Med Virol. 2019 Sep;29(5):e2073. doi: 10.1002/rmv.2073. Epub 2019 Aug 1. Rev Med Virol. 2019. PMID: 31369184 Review.
-
Interplays between Enterovirus A71 and the innate immune system.J Biomed Sci. 2019 Dec 2;26(1):95. doi: 10.1186/s12929-019-0596-8. J Biomed Sci. 2019. PMID: 31787104 Free PMC article. Review.
-
Innate Immunity Evasion by Enteroviruses: Insights into Virus-Host Interaction.Viruses. 2016 Jan 15;8(1):22. doi: 10.3390/v8010022. Viruses. 2016. PMID: 26784219 Free PMC article. Review.
-
Research progress on pathogenic and therapeutic mechanisms of Enterovirus A71.Arch Virol. 2023 Sep 29;168(10):260. doi: 10.1007/s00705-023-05882-8. Arch Virol. 2023. PMID: 37773227 Review.
-
Severe enterovirus A71 infection is associated with dysfunction of T cell immune response and alleviated by Astragaloside A.Virol Sin. 2025 Jun;40(3):451-461. doi: 10.1016/j.virs.2025.05.010. Epub 2025 May 29. Virol Sin. 2025. PMID: 40449890 Free PMC article.
Cited by
-
ILC3 Function as a Double-Edged Sword in EV71 Infection.Viruses. 2025 Jan 27;17(2):184. doi: 10.3390/v17020184. Viruses. 2025. PMID: 40006939 Free PMC article.
-
The N6-methyladenosine RNA epigenetic modification modulates the amplification of coxsackievirus B1 in human pancreatic beta cells.Front Microbiol. 2024 Dec 18;15:1501061. doi: 10.3389/fmicb.2024.1501061. eCollection 2024. Front Microbiol. 2024. PMID: 39744389 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

