Electrophysiological Effects of the Sodium-Glucose Co-Transporter-2 (SGLT2) Inhibitor Dapagliflozin on Human Cardiac Potassium Channels
- PMID: 38891889
- PMCID: PMC11172209
- DOI: 10.3390/ijms25115701
Electrophysiological Effects of the Sodium-Glucose Co-Transporter-2 (SGLT2) Inhibitor Dapagliflozin on Human Cardiac Potassium Channels
Abstract
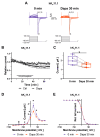
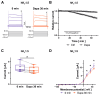
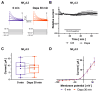
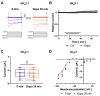
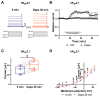
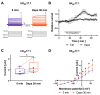
The sodium-glucose co-transporter-2 (SGLT2) inhibitor dapagliflozin is increasingly used in the treatment of diabetes and heart failure. Dapagliflozin has been associated with reduced incidence of atrial fibrillation (AF) in clinical trials. We hypothesized that the favorable antiarrhythmic outcome of dapagliflozin use may be caused in part by previously unrecognized effects on atrial repolarizing potassium (K+) channels. This study was designed to assess direct pharmacological effects of dapagliflozin on cloned ion channels Kv11.1, Kv1.5, Kv4.3, Kir2.1, K2P2.1, K2P3.1, and K2P17.1, contributing to IKur, Ito, IKr, IK1, and IK2P K+ currents. Human channels coded by KCNH2, KCNA5, KCND3, KCNJ2, KCNK2, KCNK3, and KCNK17 were heterologously expressed in Xenopus laevis oocytes, and currents were recorded using the voltage clamp technique. Dapagliflozin (100 µM) reduced Kv11.1 and Kv1.5 currents, whereas Kir2.1, K2P2.1, and K2P17.1 currents were enhanced. The drug did not significantly affect peak current amplitudes of Kv4.3 or K2P3.1 K+ channels. Biophysical characterization did not reveal significant effects of dapagliflozin on current-voltage relationships of study channels. In conclusion, dapagliflozin exhibits direct functional interactions with human atrial K+ channels underlying IKur, IKr, IK1, and IK2P currents. Substantial activation of K2P2.1 and K2P17.1 currents could contribute to the beneficial antiarrhythmic outcome associated with the drug. Indirect or chronic effects remain to be investigated in vivo.
Keywords: K+ channel; arrhythmia; dapagliflozin; ion channel; repolarization; sodium-glucose co-transporter-2 (SGLT2) inhibitor.
Conflict of interest statement
P.L. reports receiving lecture fees/honoraria from Bayer Vital, Bristol-Myers Squibb, Johnson and Johnson Medical, Boston Scientific and Pfizer Pharma. D.T. reports receiving lecture fees/honoraria from Abbott, AstraZeneca, Bayer Vital, Boehringer Ingelheim Pharma, Bristol-Myers Squibb, Daiichi Sankyo, Johnson and Johnson Medical, Medtronic, Novartis, Pfizer Pharma, Sanofi-Aventis, and ZOLL CMS. The remaining authors have reported that they have no relationships relevant to the content of this paper to disclose.
Figures

References
-
- Chugh S.S., Havmoeller R., Narayanan K., Singh D., Rienstra M., Benjamin E.J., Gillum R.F., Kim Y.H., McAnulty JHJr Zheng Z.J., Forouzanfar M.H., et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. - DOI - PMC - PubMed
-
- Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., Boriani G., Castella M., Dan G.-A., Dilaveris P.E., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- Career Development Program - Short Term Fellowship to M.E.M., Olympia Morata Grant to A.K.R./Heidelberg University
- Research Funding to D.T./Joachim Siebeneicher Foundation
- Cardiology Career Program to F.P., J.H., and H.G./Department of Cardiology Heidelberg University Hospital
- Kaltenbach-Promotionsstipendium to J.P./German Heart Foundation
- to J.H./German Academic Scholarship Foundation
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

