Reduction in Hippocampal Amyloid-β Peptide (Aβ) Content during Glycine-Proline-Glutamate (Gly-Pro-Glu) Co-Administration Is Associated with Changes in Inflammation and Insulin-like Growth Factor (IGF)-I Signaling
- PMID: 38891902
- PMCID: PMC11172028
- DOI: 10.3390/ijms25115716
Reduction in Hippocampal Amyloid-β Peptide (Aβ) Content during Glycine-Proline-Glutamate (Gly-Pro-Glu) Co-Administration Is Associated with Changes in Inflammation and Insulin-like Growth Factor (IGF)-I Signaling
Abstract
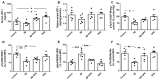
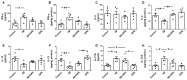
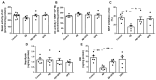
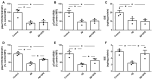
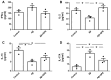
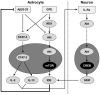
Alzheimer's disease (AD) is characterized by the deposition in the brain of senile plaques composed of amyloid-β peptides (Aβs) that increase inflammation. An endogenous peptide derived from the insulin-like growth factor (IGF)-I, glycine-proline-glutamate (GPE), has IGF-I-sensitizing and neuroprotective actions. Here, we examined the effects of GPE on Aβ levels and hippocampal inflammation generated by the intracerebroventricular infusion of Aβ25-35 for 2 weeks (300 pmol/day) in ovariectomized rats and the signaling-related pathways and levels of Aβ-degrading enzymes associated with these GPE-related effects. GPE prevented the Aβ-induced increase in the phosphorylation of p38 mitogen-activated protein kinase and the reduction in activation of signal transducer and activator of transcription 3, insulin receptor substrate-1, and Akt, as well as on interleukin (IL)-2 and IL-13 levels in the hippocampus. The functionality of somatostatin, measured as the percentage of inhibition of adenylate cyclase activity and the levels of insulin-degrading enzyme, was also preserved by GPE co-treatment. These findings indicate that GPE co-administration may protect from Aβ insult by changing hippocampal cytokine content and somatostatin functionality through regulation of leptin- and IGF-I-signaling pathways that could influence the reduction in Aβ levels through modulation of levels and/or activity of Aβ proteases.
Keywords: Alzheimer’s disease; Gly-Pro-Glu; IGF-I signaling; cytokines; inflammation.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Fornari Laurindo L., Aparecido Dias J., Cressoni Araújo A., Torres Pomini K., Machado Galhardi C., Rucco Penteado Detregiachi C., Santos de Argollo Haber L., Donizeti Roque D., Dib Bechara M., Vialogo Marques de Castro M., et al. Immunological dimensions of neuroinflammation and microglial activation: Exploring innovative immunomodulatory approaches to mitigate neuroinflammatory progression. Front. Immunol. 2024;14:1305933. doi: 10.3389/fimmu.2023.1305933. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

