Longitudinal Neuropathological Consequences of Extracranial Radiation Therapy in Mice
- PMID: 38891920
- PMCID: PMC11171684
- DOI: 10.3390/ijms25115731
Longitudinal Neuropathological Consequences of Extracranial Radiation Therapy in Mice
Abstract
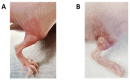
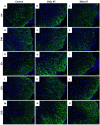
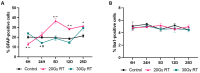
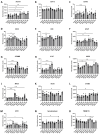
Cancer-related cognitive impairment (CRCI) is a consequence of chemotherapy and extracranial radiation therapy (ECRT). Our prior work demonstrated gliosis in the brain following ECRT in SKH1 mice. The signals that induce gliosis were unclear. Right hindlimb skin from SKH1 mice was treated with 20 Gy or 30 Gy to induce subclinical or clinical dermatitis, respectively. Mice were euthanized at 6 h, 24 h, 5 days, 12 days, and 25 days post irradiation, and the brain, thoracic spinal cord, and skin were collected. The brains were harvested for spatial proteomics, immunohistochemistry, Nanostring nCounter® glial profiling, and neuroinflammation gene panels. The thoracic spinal cords were evaluated by immunohistochemistry. Radiation injury to the skin was evaluated by histology. The genes associated with neurotransmission, glial cell activation, innate immune signaling, cell signal transduction, and cancer were differentially expressed in the brains from mice treated with ECRT compared to the controls. Dose-dependent increases in neuroinflammatory-associated and neurodegenerative-disease-associated proteins were measured in the brains from ECRT-treated mice. Histologic changes in the ECRT-treated mice included acute dermatitis within the irradiated skin of the hindlimb and astrocyte activation within the thoracic spinal cord. Collectively, these findings highlight indirect neuronal transmission and glial cell activation in the pathogenesis of ECRT-related CRCI, providing possible signaling pathways for mitigation strategies.
Keywords: SKH1 mice; cancer treatment; cancer-related cognitive impairment CRCI; neuropathology; radiation-related cognitive impairment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
The Distant Molecular Effects on the Brain by Cancer Treatment.Brain Sci. 2023 Dec 24;14(1):22. doi: 10.3390/brainsci14010022. Brain Sci. 2023. PMID: 38248237 Free PMC article.
-
Cancer treatment induces neuroinflammation and behavioral deficits in mice.Front Behav Neurosci. 2023 Jan 9;16:1067298. doi: 10.3389/fnbeh.2022.1067298. eCollection 2022. Front Behav Neurosci. 2023. PMID: 36699654 Free PMC article.
-
Radiation Disrupts the Protective Function of the Spinal Meninges in a Mouse Model of Tumor-induced Spinal Cord Compression.Clin Orthop Relat Res. 2021 Jan 1;479(1):163-176. doi: 10.1097/CORR.0000000000001449. Clin Orthop Relat Res. 2021. PMID: 32858719 Free PMC article.
-
Glial cell response after aneurysmal subarachnoid hemorrhage - Functional consequences and clinical implications.Biochim Biophys Acta. 2016 Mar;1862(3):492-505. doi: 10.1016/j.bbadis.2015.10.013. Epub 2015 Oct 19. Biochim Biophys Acta. 2016. PMID: 26493445 Review. No abstract available.
-
Glial-glial and glial-neuronal interfaces in radiation-induced, glia-depleted spinal cord.J Anat. 1997 Jan;190 ( Pt 1)(Pt 1):5-21. doi: 10.1046/j.1469-7580.1997.19010005.x. J Anat. 1997. PMID: 9034878 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

