Granulocyte Colony Stimulating Factor-Mobilized Peripheral Blood Mononuclear Cells: An Alternative Cellular Source for Chimeric Antigen Receptor Therapy
- PMID: 38891957
- PMCID: PMC11171785
- DOI: 10.3390/ijms25115769
Granulocyte Colony Stimulating Factor-Mobilized Peripheral Blood Mononuclear Cells: An Alternative Cellular Source for Chimeric Antigen Receptor Therapy
Abstract
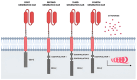
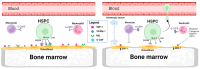
Lymphocyte collection by apheresis for CAR-T production usually does not include blood mobilized using granulocyte colony stimulating factor (G-CSF) due to the widespread knowledge that it causes a decrease in the number and functionality of lymphocytes. However, it is used for stem cell transplant, which is a common treatment for hematological malignancies. The growing demand for CAR therapies (CAR-T and NK-CAR), both in research and clinics, makes it necessary to evaluate whether mobilized PBSC products may be potential candidates for use in such therapies. This review collects recent works that experimentally verify the role and functionality of T and NK lymphocytes and the generation of CAR-T from apheresis after G-CSF mobilization. As discussed, T cells do not vary significantly in their phenotype, the ratio of CD4+ and CD8+ remains constant, and the different sub-populations remain stable. In addition, the expansion and proliferation rates are invariant regardless of mobilization with G-CSF as well as the secretion of proinflammatory cytokines and the cytotoxic ability. Therefore, cells mobilized before apheresis are postulated as a new alternative source of T cells for adoptive therapies that will serve to alleviate high demand, increase availability, and take advantage of the substantial number of existing cryopreserved products.
Keywords: CAR-NK; CAR-T; G-CSF; mobilization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Modification of NK cell subset repartition and functions in granulocyte colony-stimulating factor-mobilized leukapheresis after expansion with IL-15.Immunol Res. 2017 Dec;65(6):1130-1138. doi: 10.1007/s12026-017-8955-6. Immunol Res. 2017. PMID: 29019081
-
Manufacture of Chimeric Antigen Receptor T Cells from Mobilized Cyropreserved Peripheral Blood Stem Cell Units Depends on Monocyte Depletion.Biol Blood Marrow Transplant. 2019 Feb;25(2):223-232. doi: 10.1016/j.bbmt.2018.10.004. Epub 2018 Oct 10. Biol Blood Marrow Transplant. 2019. PMID: 30315942
-
Manufacturing of highly functional and specific T cells for adoptive immunotherapy against virus from granulocyte colony-stimulating factor-mobilized donors.Cytotherapy. 2014 Oct;16(10):1390-408. doi: 10.1016/j.jcyt.2014.05.009. Epub 2014 Jun 18. Cytotherapy. 2014. PMID: 24954783
-
Immunologic profiles of effector cells and peripheral blood stem cells mobilized with different hematopoietic growth factors.Stem Cells. 2000;18(6):390-8. doi: 10.1634/stemcells.18-6-390. Stem Cells. 2000. PMID: 11072026 Review.
-
[Allogeneic CAR-NK cells: A promising alternative to autologous CAR-T cells - State of the art, sources of NK cells, limits and perspectives].Bull Cancer. 2021 Oct;108(10S):S81-S91. doi: 10.1016/j.bulcan.2021.06.007. Bull Cancer. 2021. PMID: 34920811 Review. French.
References
-
- Minda A.G., Awel F.S., Seifudin K.A., Gezahegne M.K. Immunotherapy against Cancer: A Comprehensive Review. J. Cancer Res. Exp. Oncol. 2016;8:15–25. doi: 10.5897/jcreo2015.0124. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

