Revolutionizing Glioblastoma Treatment: A Comprehensive Overview of Modern Therapeutic Approaches
- PMID: 38891962
- PMCID: PMC11172387
- DOI: 10.3390/ijms25115774
Revolutionizing Glioblastoma Treatment: A Comprehensive Overview of Modern Therapeutic Approaches
Abstract
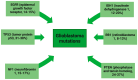
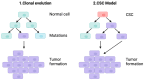
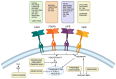
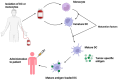
Glioblastoma is the most common malignant primary brain tumor in the adult population, with an average survival of 12.1 to 14.6 months. The standard treatment, combining surgery, radiotherapy, and chemotherapy, is not as efficient as we would like. However, the current possibilities are no longer limited to the standard therapies due to rapid advancements in biotechnology. New methods enable a more precise approach by targeting individual cells and antigens to overcome cancer. For the treatment of glioblastoma, these are gamma knife therapy, proton beam therapy, tumor-treating fields, EGFR and VEGF inhibitors, multiple RTKs inhibitors, and PI3K pathway inhibitors. In addition, the increasing understanding of the role of the immune system in tumorigenesis and the ability to identify tumor-specific antigens helped to develop immunotherapies targeting GBM and immune cells, including CAR-T, CAR-NK cells, dendritic cells, and immune checkpoint inhibitors. Each of the described methods has its advantages and disadvantages and faces problems, such as the inefficient crossing of the blood-brain barrier, various neurological and systemic side effects, and the escape mechanism of the tumor. This work aims to present the current modern treatments of glioblastoma.
Keywords: CAR-T; cancer; genetics; glioblastoma; immune checkpoint inhibitors; immunotherapy.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Sloan E.A., Hilz S., Gupta R., Cadwell C., Ramani B., Hofmann J., Kline C.N., Banerjee A., Reddy A., Bush N.A.O., et al. Gliomas arising in the setting of Li-Fraumeni syndrome stratify into two molecular subgroups with divergent clinicopathologic features. Acta Neuropathol. 2020;139:953–957. doi: 10.1007/s00401-020-02144-8. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

