Structural Features Influencing the Bioactive Conformation of Angiotensin II and Angiotensin A: Relationship between Receptor Desensitization, Addiction, and the Blood-Brain Barrier
- PMID: 38891966
- PMCID: PMC11171751
- DOI: 10.3390/ijms25115779
Structural Features Influencing the Bioactive Conformation of Angiotensin II and Angiotensin A: Relationship between Receptor Desensitization, Addiction, and the Blood-Brain Barrier
Abstract
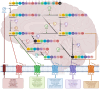
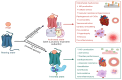
The N-terminal portion of the octapeptide angiotensin II (DRVYIHPF; AngII), a vasopressor peptide that favorably binds to, and activates, AngII type 1 receptor (AT1R), has an important role in maintaining bioactive conformation. It involves all three charged groups, namely (i) the N-terminal amino group cation, (ii) the Asp sidechain anion and (iii) the Arg guanidino cation. Neutralization of any one of these three charged groups results in a substantial reduction (<5%) in bioactivity, implicating a specialized function for this cluster. In contrast, angiotensin A (ARVYIHPF; AngA) has reduced bioactivity at AT1R; however, replacement of Asp in AngII with sarcosine (N-methyl-glycine) not only restores bioactivity but increases the activity of agonist, antagonist, and inverse agonist analogues. A bend produced at the N-terminus by the introduction of the secondary amino acid sarcosine is thought to realign the functional groups that chaperone the C-terminal portion of AngII, allowing transfer of the negative charge originating at the C-terminus to be transferred to the Tyr hydroxyl-forming tyrosinate anion, which is required to activate the receptor and desensitizes the receptor (tachyphylaxis). Peptide (sarilesin) and nonpeptide (sartans) moieties, which are long-acting inverse agonists, appear to desensitize the receptor by a mechanism analogous to tachyphylaxis. Sartans/bisartans were found to bind to alpha adrenergic receptors resulting in structure-dependent desensitization or resensitization. These considerations have provided information on the mechanisms of receptor desensitization/tolerance and insights into possible avenues for treating addiction. In this regard sartans, which appear to cross the blood-brain barrier more readily than bisartans, are the preferred drug candidates.
Keywords: G-protein coupled receptor; addiction; angiotensin II; arginine; bisartan; blood–brain barrier; conformation of angiotensin; coronavirus disease 2019; receptor desensitization.
Conflict of interest statement
A.Z. co-owns Zultek Engineering, the provider of product OB16 organ bath systems. Authors J.M.M. and K.K. were employed by NewDrug and author G.J.M. by Pepmetics Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Fluyau D., Hashmi M.F., Charlton T.E. Drug Addiction. StatPearls; Treasure Island, FL, USA: 2024. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

