Modulation of the p75NTR during Adolescent Alcohol Exposure Prevents Cholinergic Neuronal Atrophy and Associated Acetylcholine Activity and Behavioral Dysfunction
- PMID: 38891978
- PMCID: PMC11172149
- DOI: 10.3390/ijms25115792
Modulation of the p75NTR during Adolescent Alcohol Exposure Prevents Cholinergic Neuronal Atrophy and Associated Acetylcholine Activity and Behavioral Dysfunction
Abstract
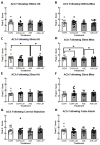
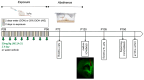
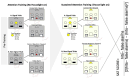
Binge alcohol consumption during adolescence can produce lasting deficits in learning and memory while also increasing the susceptibility to substance use disorders. The adolescent intermittent ethanol (AIE) rodent model mimics human adolescent binge drinking and has identified the nucleus basalis magnocellularis (NbM) as a key site of pathology. The NbM is a critical regulator of prefrontal cortical (PFC) cholinergic function and attention. The cholinergic phenotype is controlled pro/mature neurotrophin receptor activation. We sought to determine if p75NTR activity contributes to the loss of cholinergic phenotype in AIE by using a p75NTR modulator (LM11A-31) to inhibit prodegenerative signaling during ethanol exposure. Male and female rats underwent 5 g/kg ethanol (AIE) or water (CON) exposure following 2-day-on 2-day-off cycles from postnatal day 25-57. A subset of these groups also received a protective dose of LM11A-31 (50 mg/kg) during adolescence. Rats were trained on a sustained attention task (SAT) and behaviorally relevant acetylcholine (ACh) activity was recorded in the PFC with a fluorescent indicator (AChGRAB 3.0). AIE produced learning deficits on the SAT, which were spared with LM11A-31. In addition, PFC ACh activity was blunted by AIE, which LM11A-31 corrected. Investigation of NbM ChAT+ and TrkA+ neuronal expression found that AIE led to a reduction of ChAT+TrkA+ neurons, which again LM11A-31 protected. Taken together, these findings demonstrate the p75NTR activity during AIE treatment is a key regulator of cholinergic degeneration.
Keywords: acetylcholine; adolescence; alcohol; basal forebrain; frontal cortex; neurotrophin.
Conflict of interest statement
The authors declare not conflict of interest.
Figures







Update of
-
Modulation of the p75NTR during adolescent alcohol exposure prevents cholinergic neuronal atrophy and associated acetylcholine activity and behavioral dysfunction.bioRxiv [Preprint]. 2024 Apr 5:2024.04.03.587970. doi: 10.1101/2024.04.03.587970. bioRxiv. 2024. Update in: Int J Mol Sci. 2024 May 26;25(11):5792. doi: 10.3390/ijms25115792. PMID: 38617368 Free PMC article. Updated. Preprint.
References
-
- Crews F.T., Robinson D.L., Chandler L.J., Ehlers C.L., Mulholland P.J., Pandey S.C., Rodd Z.A., Spear L.P., Swartzwelder H.S., Vetreno R.P. Mechanisms of Persistent Neurobiological Changes Following Adolescent Alcohol Exposure: NADIA Consortium Findings. Alcohol. Clin. Exp. Res. 2019;43:1806–1822. doi: 10.1111/acer.14154. - DOI - PMC - PubMed
-
- Záborszky L., Gombkoto P., Varsanyi P., Gielow M.R., Poe G., Role L.W., Ananth M., Rajebhosale P., Talmage D.A., Hasselmo M.E., et al. Specific Basal Forebrain-Cortical Cholinergic Circuits Coordinate Cognitive Operations. J. Neurosci. 2018;38:9446–9458. doi: 10.1523/jneurosci.1676-18.2018. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

