Studying the Effect of the Host Genetic Background of Juvenile Polyposis Development Using Collaborative Cross and Smad4 Knock-Out Mouse Models
- PMID: 38891999
- PMCID: PMC11172477
- DOI: 10.3390/ijms25115812
Studying the Effect of the Host Genetic Background of Juvenile Polyposis Development Using Collaborative Cross and Smad4 Knock-Out Mouse Models
Abstract
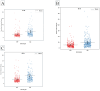
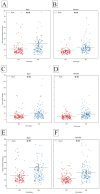
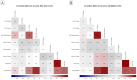
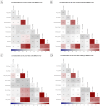
Juvenile polyposis syndrome (JPS) is a rare autosomal dominant disorder characterized by multiple juvenile polyps in the gastrointestinal tract, often associated with mutations in genes such as Smad4 and BMPR1A. This study explores the impact of Smad4 knock-out on the development of intestinal polyps using collaborative cross (CC) mice, a genetically diverse model. Our results reveal a significant increase in intestinal polyps in Smad4 knock-out mice across the entire population, emphasizing the broad influence of Smad4 on polyposis. Sex-specific analyses demonstrate higher polyp counts in knock-out males and females compared to their WT counterparts, with distinct correlation patterns. Line-specific effects highlight the nuanced response to Smad4 knock-out, underscoring the importance of genetic variability. Multimorbidity heat maps offer insights into complex relationships between polyp counts, locations, and sizes. Heritability analysis reveals a significant genetic basis for polyp counts and sizes, while machine learning models, including k-nearest neighbors and linear regression, identify key predictors, enhancing our understanding of juvenile polyposis genetics. Overall, this study provides new information on understanding the intricate genetic interplay in the context of Smad4 knock-out, offering valuable insights that could inform the identification of potential therapeutic targets for juvenile polyposis and related diseases.
Keywords: Smad4; collaborative cross mice; intestinal cancer; intestinal polyps; juvenile polyposis syndrome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
A novel germline mutation in exon 10 of the SMAD4 gene in a familial juvenile polyposis.Gut Liver. 2013 Nov;7(6):747-51. doi: 10.5009/gnl.2013.7.6.747. Epub 2013 Nov 11. Gut Liver. 2013. PMID: 24312718 Free PMC article.
-
Phenotypic Differences in Juvenile Polyposis Syndrome With or Without a Disease-causing SMAD4/BMPR1A Variant.Cancer Prev Res (Phila). 2021 Feb;14(2):215-222. doi: 10.1158/1940-6207.CAPR-20-0348. Epub 2020 Oct 23. Cancer Prev Res (Phila). 2021. PMID: 33097490 Free PMC article.
-
Juvenile polyposis syndrome might be misdiagnosed as familial adenomatous polyposis: a case report and literature review.BMC Gastroenterol. 2020 Jun 1;20(1):167. doi: 10.1186/s12876-020-01238-7. BMC Gastroenterol. 2020. PMID: 32487124 Free PMC article. Review.
-
Somatic alterations in juvenile polyps from BMPR1A and SMAD4 mutation carriers.Genes Chromosomes Cancer. 2015 Sep;54(9):575-82. doi: 10.1002/gcc.22270. Epub 2015 Jul 14. Genes Chromosomes Cancer. 2015. PMID: 26171675
-
Juvenile polyposis syndrome.World J Gastroenterol. 2011 Nov 28;17(44):4839-44. doi: 10.3748/wjg.v17.i44.4839. World J Gastroenterol. 2011. PMID: 22171123 Free PMC article. Review.
Cited by
-
The collaborative cross mouse for studying the effect of host genetic background on memory impairments due to obesity and diabetes.Animal Model Exp Med. 2025 Jan;8(1):126-141. doi: 10.1002/ame2.12488. Epub 2024 Oct 28. Animal Model Exp Med. 2025. PMID: 39468690 Free PMC article.
-
System genetic analysis of intestinal cancer and periodontitis development as influenced by aging and diabesity using Collaborative Cross mice.Animal Model Exp Med. 2025 Apr;8(4):758-770. doi: 10.1002/ame2.12568. Epub 2025 Feb 7. Animal Model Exp Med. 2025. PMID: 39921239 Free PMC article. Review.
References
-
- Van Hattem W.A., Langeveld D., De Leng W.W.J., Morsink F.H., Van Diest P.J., Iacobuzio-Donahue C.A., Giardiello F.M., Offerhaus G.J.A., Brosens L.A.A. Histological Variations in Juvenile Polyp Phenotype Correlate with Genetic Defect Underlying Juvenile Polyposis. Am. J. Surg. Pathol. 2011;35:530. doi: 10.1097/PAS.0B013E318211CAE1. - DOI - PMC - PubMed
-
- Blatter R., Tschupp B., Aretz S., Bernstein I., Colas C., Evans D.G., Genuardi M., Hes F.J., Hüneburg R., Järvinen H., et al. Disease Expression in Juvenile Polyposis Syndrome: A Retrospective Survey on a Cohort of 221 European Patients and Comparison with a Literature-Derived Cohort of 473 SMAD4/BMPR1A Pathogenic Variant Carriers. Genet. Med. 2020;22:1524–1532. doi: 10.1038/s41436-020-0826-1. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

