A Pilot Study on Circulating, Cellular, and Tissue Biomarkers in Osteosarcopenic Patients
- PMID: 38892069
- PMCID: PMC11172451
- DOI: 10.3390/ijms25115879
A Pilot Study on Circulating, Cellular, and Tissue Biomarkers in Osteosarcopenic Patients
Abstract
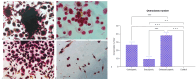
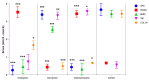
Aging comes with the loss of muscle and bone mass, leading to a condition known as osteosarcopenia. Circulating, cellular, and tissue biomarkers research for osteosarcopenia is relatively scarce and, currently, no established biomarkers exist. Here we find that osteosarcopenic patients exhibited elevated basophils and TNFα levels, along with decreased aPPT, PT/INR, IL15, alpha-Klotho, DHEA-S, and FGF-2 expression and distinctive bone and muscle tissue micro-architecture and biomarker expressions. They also displayed an increase in osteoclast precursors with a concomitant imbalance towards spontaneous osteoclastogenesis. Similarities were noted with osteopenic and sarcopenic patients, including a lower neutrophil percentage and altered cytokine expression. A linear discriminant analysis (LDA) on models based on selected biomarkers showed a classification accuracy in the range of 61-78%. Collectively, our data provide compelling evidence for novel biomarkers for osteosarcopenia that may hold potential as diagnostic tools to promote healthy aging.
Keywords: aging; biomarkers; osteopenia; osteosarcopenia; sarcopenia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

