Anti-HMGB1 mAb Therapy Reduces Epidural Hematoma Injury
- PMID: 38892076
- PMCID: PMC11172231
- DOI: 10.3390/ijms25115889
Anti-HMGB1 mAb Therapy Reduces Epidural Hematoma Injury
Abstract
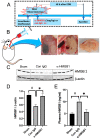
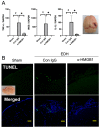
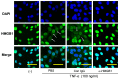
Epidural and subdural hematomas are commonly associated with traumatic brain injury. While surgical removal is the primary intervention for these hematomas, it is also critical to prevent and reduce complications such as post-traumatic epilepsy, which may result from inflammatory responses in the injured brain areas. In the present study, we observed that high mobility group box-1 (HMGB1) decreased in the injured brain area beneath the epidural hematoma (EDH) in rats, concurrent with elevated plasma levels of HMGB1. Anti-HMGB1 monoclonal antibody therapy strongly inhibited both HMGB1 release and the subsequent increase in plasma levels. Moreover, this treatment suppressed the up-regulation of inflammatory cytokines and related molecules such as interleukin-1-beta (IL-1β), tumor necrosis factor-alpha (TNF-α), and inducible nitric oxide synthase (iNOS) in the injured areas. Our in vitro experiments using SH-SY5Y demonstrated that hematoma components-thrombin, heme, and ferrous ion- prompted HMGB1 translocation from the nuclei to the cytoplasm, a process inhibited by the addition of the anti-HMGB1 mAb. These findings suggest that anti-HMGB1 mAb treatment not only inhibits HMGB1 translocation but also curtails inflammation in injured areas, thereby protecting the neural tissue. Thus, anti-HMGB1 mAb therapy could serve as a complementary therapy for an EDH before/after surgery.
Keywords: HMGB1; epidural hematoma; inflammatory response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

